Real time monitoring of endogenous cytoplasmic mRNA using linear antisense 2'-O-methyl RNA probes in living cells
- PMID: 21106497
- PMCID: PMC3045578
- DOI: 10.1093/nar/gkq1196
Real time monitoring of endogenous cytoplasmic mRNA using linear antisense 2'-O-methyl RNA probes in living cells
Abstract
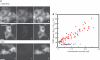
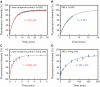
Visualization and monitoring of endogenous mRNA in the cytoplasm of living cells promises a significant comprehension of refined post-transcriptional regulation. Fluorescently labeled linear antisense oligonucleotides can bind to natural mRNA in a sequence-specific way and, therefore, provide a powerful tool in probing endogenous mRNA. Here, we investigated the feasibility of using linear antisense probes to monitor the variable and dynamic expression of endogenous cytoplasmic mRNAs. Two linear antisense 2'-O-methyl RNA probes, which have different interactive fluorophores at the 5'-end of one probe and at the 3'-end of the other, were used to allow fluorescence resonance energy transfer (FRET) upon hybridization to the target mRNA. By characterizing the formation of the probe-mRNA hybrids in living cells, we found that the probe composition and concentration are crucial parameters in the visualization of endogenous mRNA with high specificity. Furthermore, rapid hybridization (within 1 min) of the linear antisense probe enabled us to visualize dynamic processes of endogenous c-fos mRNA, such as fast elevation of levels after gene induction and the localization of c-fos mRNA in stress granules in response to cellular stress. Thus, our approach provides a basis for real time monitoring of endogenous cytoplasmic mRNA in living cells.
Figures




References
-
- Anderson P, Kedarsha N. RNA granules: post transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 2009;10:430–436. - PubMed
-
- Bushati N, Cohen SM. microRNA functions. Annu. Rev. Cell Dev. Biol. 2009;23:75–105. - PubMed
-
- Chekulaeva M, Filipowicz W. Mechanism of miRNA-mediated post-transcriptional regulation in animal cells. Curr. Opin. Cell Biol. 2009;21:452–460. - PubMed
-
- Dirks RW, Tanke HJ. Advances in fluorescent tracking of nucleic acids in living cells. Biotechniques. 2006;40:489–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

