Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning
- PMID: 21106527
- PMCID: PMC3030352
- DOI: 10.1074/jbc.M110.161109
Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning
Abstract
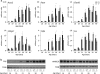
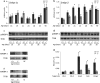
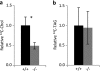
The acyl-CoA-binding protein (ACBP)/diazepam binding inhibitor is an intracellular protein that binds C(14)-C(22) acyl-CoA esters and is thought to act as an acyl-CoA transporter. In vitro analyses have indicated that ACBP can transport acyl-CoA esters between different enzymatic systems; however, little is known about the in vivo function in mammalian cells. We have generated mice with targeted disruption of ACBP (ACBP(-/-)). These mice are viable and fertile and develop normally. However, around weaning, the ACBP(-/-) mice go through a crisis with overall weakness and a slightly decreased growth rate. Using microarray analysis, we show that the liver of ACBP(-/-) mice displays a significantly delayed adaptation to weaning with late induction of target genes of the sterol regulatory element-binding protein (SREBP) family. As a result, hepatic de novo cholesterogenesis is decreased at weaning. The delayed induction of SREBP target genes around weaning is caused by a compromised processing and decreased expression of SREBP precursors, leading to reduced binding of SREBP to target sites in chromatin. In conclusion, lack of ACBP interferes with the normal metabolic adaptation to weaning and leads to delayed induction of the lipogenic gene program in the liver.
Figures






Similar articles
-
Delayed hepatic adaptation to weaning in ACBP-/- mice is caused by disruption of the epidermal barrier.Cell Rep. 2013 Dec 12;5(5):1403-12. doi: 10.1016/j.celrep.2013.11.010. Epub 2013 Dec 5. Cell Rep. 2013. PMID: 24316079
-
Downregulation of PPARs and SREBP by acyl-CoA-binding protein overexpression in transgenic rats.Pflugers Arch. 2008 May;456(2):369-77. doi: 10.1007/s00424-007-0416-y. Epub 2007 Dec 22. Pflugers Arch. 2008. PMID: 18157544
-
The gene encoding acyl-CoA-binding protein is subject to metabolic regulation by both sterol regulatory element-binding protein and peroxisome proliferator-activated receptor alpha in hepatocytes.J Biol Chem. 2005 Feb 18;280(7):5258-66. doi: 10.1074/jbc.M407515200. Epub 2004 Dec 15. J Biol Chem. 2005. PMID: 15611101
-
The function of acyl-CoA-binding protein (ACBP)/diazepam binding inhibitor (DBI).Mol Cell Biochem. 1993 Jun 9-23;123(1-2):129-38. doi: 10.1007/BF01076484. Mol Cell Biochem. 1993. PMID: 8232254 Review.
-
Acyl-CoA-binding protein (ACBP) and its relation to fatty acid-binding protein (FABP): an overview.Mol Cell Biochem. 1990 Oct 15-Nov 8;98(1-2):217-23. doi: 10.1007/BF00231387. Mol Cell Biochem. 1990. PMID: 2266962 Review.
Cited by
-
Subregion-Specific Impacts of Genetic Loss of Diazepam Binding Inhibitor on Synaptic Inhibition in the Murine Hippocampus.Neuroscience. 2018 Sep 15;388:128-138. doi: 10.1016/j.neuroscience.2018.07.012. Epub 2018 Jul 19. Neuroscience. 2018. PMID: 30031126 Free PMC article.
-
Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis.Endocrinology. 2014 Jan;155(1):89-97. doi: 10.1210/en.2013-1556. Epub 2013 Dec 20. Endocrinology. 2014. PMID: 24174323 Free PMC article.
-
The Gliopeptide ODN, a Ligand for the Benzodiazepine Site of GABAA Receptors, Boosts Functional Recovery after Stroke.J Neurosci. 2021 Aug 18;41(33):7148-7159. doi: 10.1523/JNEUROSCI.2255-20.2021. Epub 2021 Jul 1. J Neurosci. 2021. PMID: 34210784 Free PMC article.
-
A brief history of the search for the protein(s) involved in the acute regulation of steroidogenesis.Mol Cell Endocrinol. 2017 Feb 5;441:7-16. doi: 10.1016/j.mce.2016.07.036. Epub 2016 Jul 30. Mol Cell Endocrinol. 2017. PMID: 27484452 Free PMC article. Review.
-
The acyl-CoA binding protein is required for normal epidermal barrier function in mice.J Lipid Res. 2012 Oct;53(10):2162-2174. doi: 10.1194/jlr.M029553. Epub 2012 Jul 24. J Lipid Res. 2012. PMID: 22829653 Free PMC article.
References
-
- Faergeman N. J., Wadum M., Feddersen S., Burton M., Kragelund B. B., Knudsen J. (2007) Mol. Cell. Biochem. 299, 55–65 - PubMed
-
- Mandrup S., Andreasen P. H., Knudsen J., Kristiansen K. (1993) Mol. Cell. Biochem. 123, 55–61 - PubMed
-
- Mandrup S., Hummel R., Ravn S., Jensen G., Andreasen P. H., Gregersen N., Knudsen J., Kristiansen K. (1992) J. Mol. Biol. 228, 1011–1022 - PubMed
-
- Neess D., Kiilerich P., Sandberg M. B., Helledie T., Nielsen R., Mandrup S. (2006) Mol. Cell. Biochem. 284, 149–157 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

