Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system
- PMID: 21106539
- PMCID: PMC3030347
- DOI: 10.1074/jbc.M110.190637
Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system
Abstract
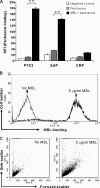
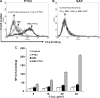
The long pentraxin 3 (PTX3), serum amyloid P component (SAP), and C-reactive protein belong to the pentraxin family of pattern recognition molecules involved in tissue homeostasis and innate immunity. They interact with C1q from the classical complement pathway. Whether this also occurs via the analogous mannose-binding lectin (MBL) from the lectin complement pathway is unknown. Thus, we investigated the possible interaction between MBL and the pentraxins. We report that MBL bound PTX3 and SAP partly via its collagen-like domain but not C-reactive protein. MBL-PTX3 complex formation resulted in recruitment of C1q, but this was not seen for the MBL-SAP complex. However, both MBL-PTX3 and MBL-SAP complexes enhanced C4 and C3 deposition and opsonophagocytosis of Candida albicans by polymorphonuclear leukocytes. Interaction between MBL and PTX3 led to communication between the lectin and classical complement pathways via recruitment of C1q, whereas SAP-enhanced complement activation occurs via a hitherto unknown mechanism. Taken together, MBL-pentraxin heterocomplexes trigger cross-activation of the complement system.
Figures








Similar articles
-
PTX3 as a paradigm for the interaction of pentraxins with the complement system.Semin Immunol. 2013 Feb;25(1):79-85. doi: 10.1016/j.smim.2013.05.002. Epub 2013 Jun 6. Semin Immunol. 2013. PMID: 23747040 Review.
-
Interactions of the humoral pattern recognition molecule PTX3 with the complement system.Immunobiology. 2012 Nov;217(11):1122-8. doi: 10.1016/j.imbio.2012.07.004. Immunobiology. 2012. PMID: 22964239 Review.
-
Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation.Biochemistry. 2006 Sep 26;45(38):11540-51. doi: 10.1021/bi0607453. Biochemistry. 2006. PMID: 16981714
-
The tissue pentraxin PTX3 limits C1q-mediated complement activation and phagocytosis of apoptotic cells by dendritic cells.J Leukoc Biol. 2006 Jul;80(1):87-95. doi: 10.1189/jlb.0805445. Epub 2006 Apr 14. J Leukoc Biol. 2006. PMID: 16617159
-
Pentraxins in Complement Activation and Regulation.Front Immunol. 2018 Dec 19;9:3046. doi: 10.3389/fimmu.2018.03046. eCollection 2018. Front Immunol. 2018. PMID: 30619374 Free PMC article. Review.
Cited by
-
Biology of human pentraxin 3 (PTX3) in acute and chronic kidney disease.J Clin Immunol. 2013 Jul;33(5):881-90. doi: 10.1007/s10875-013-9879-0. Epub 2013 Feb 27. J Clin Immunol. 2013. PMID: 23443958 Review.
-
The Influence of Pentraxin 3 on the Ovarian Function and Its Impact on Fertility.Front Immunol. 2018 Nov 29;9:2808. doi: 10.3389/fimmu.2018.02808. eCollection 2018. Front Immunol. 2018. PMID: 30555480 Free PMC article. Review.
-
New Insights on the Role of pentraxin-3 in Allergic Asthma.Front Allergy. 2021 Jun 11;2:678023. doi: 10.3389/falgy.2021.678023. eCollection 2021. Front Allergy. 2021. PMID: 35387000 Free PMC article. Review.
-
Human serum proteins bind to Sporothrix schenckii conidia with differential effects on phagocytosis.Braz J Microbiol. 2021 Mar;52(1):33-39. doi: 10.1007/s42770-020-00276-3. Epub 2020 May 7. Braz J Microbiol. 2021. PMID: 32382937 Free PMC article.
-
Complementary Roles of Short and Long Pentraxins in the Complement-Mediated Immune Response to Aspergillus fumigatus Infections.Front Immunol. 2021 Nov 18;12:785883. doi: 10.3389/fimmu.2021.785883. eCollection 2021. Front Immunol. 2021. PMID: 34868070 Free PMC article. Review.
References
-
- Garred P., Larsen F., Seyfarth J., Fujita R., Madsen H. O. (2006) Genes Immun 7, 85–94 - PubMed
-
- Thiel S., Vorup-Jensen T., Stover C. M., Schwaeble W., Laursen S. B., Poulsen K., Willis A. C., Eggleton P., Hansen S., Holmskov U., Reid K. B., Jensenius J. C. (1997) Nature 386, 506–510 - PubMed
-
- Chen C. B., Wallis R. (2004) J. Biol. Chem. 279, 26058–26065 - PubMed
-
- Takahashi M., Iwaki D., Kanno K., Ishida Y., Xiong J., Matsushita M., Endo Y., Miura S., Ishii N., Sugamura K., Fujita T. (2008) J. Immunol. 180, 6132–6138 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

