Substrate-induced phenotypic switches of human smooth muscle cells: an in vitro study of in-stent restenosis activation pathways
- PMID: 21106574
- PMCID: PMC3061099
- DOI: 10.1098/rsif.2010.0532
Substrate-induced phenotypic switches of human smooth muscle cells: an in vitro study of in-stent restenosis activation pathways
Abstract
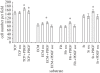
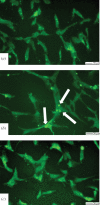
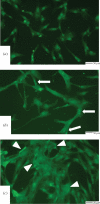
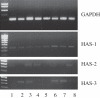
In-stent restenosis is a clinical complication following coronary angioplasty caused by the implantation of the metal device in the atherosclerotic vessel. Histological examination has shown a clear contribution of both inflammatory and smooth muscle cells (SMCs) to the deposition of an excess of neointimal tissue. However, the sequence of events leading to clinically relevant restenosis is unknown. This paper aims to study the phenotype of SMCs when adhering on substrates and exposed to biochemical stimuli typical of the early phases of stent implantation. In particular, human SMC phenotype was studied when adhering on extracellular matrix-like material (collagen-rich gel), thrombus-like material (fibrin gel) and stent material (stainless steel) in the presence or absence of a platelet-derived growth factor (PDGF) stimulus. Cells on the collagen and fibrin-rich substrates maintained their contractile phenotype. By contrast, cells on stainless steel acquired a secretory phenotype with a proliferation rate 50 per cent higher than cells on the natural substrates. Cells on stainless steel also showed an increase in PDGF-BB receptor expression, thus explaining the increase in proliferation observed when cells were subject to PDGF-BB stimuli. The stainless steel substrate also promoted a different pattern of β1-integrin localization and an altered expression of hyaluronan (HA) synthase isoforms where the synthesis of high-molecular-weight HA seemed to be favoured. These findings highlighted the induction of a phenotypic pattern in SMC by the stainless steel substrate whereby the formation of a HA-rich neointimal tissue is enhanced.
Figures

References
-
- Santin M., Colombo P., Bruschi G. 2005. Interfacial biology of in-stent restonosis. Exp. Rev. Med. Dev. 2, 429–443 10.1586/17434440.2.4.429 (doi:10.1586/17434440.2.4.429) - DOI - PubMed
-
- Farb A., Sangiorgi G., Carter A. J., Walley V. M., Edwards W. D., Schwartz R. S., Virmani R. 1999. Pathology of acute and chronic coronary stenting in humans. Circulation 99, 44–52 - PubMed
-
- McFadden E., et al. 2004. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 364, 1519–1521 10.1016/S0140-6736(04)17275-9 (doi:10.1016/S0140-6736(04)17275-9) - DOI - PubMed
-
- Burke A. P., Kolodgie F. D., Zieske A., Fowler D. R., Weber D. K., Varghese P. J., Farb A., Virmani R. 2004. Morphologic findings of coronary atherosclerotic plaques in diabetics: a post-mortem study. Arterioscler. Thromb. Vasc. Biol. 24, 1266–1271 10.1161/01.ATV.0000131783.74034.97 (doi:10.1161/01.ATV.0000131783.74034.97) - DOI - PubMed
-
- Farb A., Weber D. K., Kolodgie F. D., Burke A. P., Virmani R. 2002. Morphological predictors of restenosis after coronary stenting in humans. Circulation 105, 2974–2980 10.1161/01.CIR.0000019071.72887.BD (doi:10.1161/01.CIR.0000019071.72887.BD) - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
