Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial
- PMID: 21106618
- PMCID: PMC2991241
- DOI: 10.1136/bmj.c6325
Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial
Abstract
Objectives: To evaluate the effectiveness of integrated motivational interviewing and cognitive behavioural therapy in addition to standard care for patients with psychosis and a comorbid substance use problem.
Design: Two centre, open, rater blind randomised controlled trial.
Setting: Secondary care in the United Kingdom.
Participants: 327 patients with a clinical diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder and a diagnosis of dependence on or misuse of drugs, alcohol, or both according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
Intervention: The intervention was integrated motivational interviewing and cognitive behavioural therapy plus standard care, which was compared with standard care alone. Phase one of therapy-"motivation building"-concerns engaging the patient, then exploring and resolving ambivalence for change in substance use. Phase two-"action"-supports and facilitates change using cognitive behavioural approaches. Up to 26 therapy sessions were delivered over one year.
Main outcome measures: The primary outcome was death from any cause or admission to hospital in the 12 months after completion of therapy. Secondary outcomes were frequency and amount of substance use (assessed using the timeline followback method), readiness to change, perceived negative consequences of use, psychotic symptom ratings, number and duration of relapses, and global assessment of functioning and deliberate self harm at 12 and 24 months, with additional timeline followback assessments at 6 and 18 months. Analysis was by intention to treat and robust treatment effect estimates were produced.
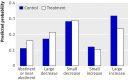
Results: 327 participants were randomly allocated to either the intervention (n=164) or treatment as usual (n=163). At 24 months, 326 (99.7%) were assessed on the primary outcome and 246 (75.2%) on the main secondary outcomes. Treatment had no beneficial effect on hospital admissions or death during follow-up, with 23.3% (38/163) of the therapy group and 20.2% (33/163) of controls deceased or admitted (adjusted odds ratio 1.16, 95% confidence interval 0.68 to 1.99; P=0.579). Therapy had no effect on the frequency of substance use or the perceived negative consequences of misuse, but did have a statistically significant effect on amount used per substance using day (adjusted ORs for main substance 1.50, 95% CI 1.08 to 2.09; P=0.016; and all substances 1.48, 95% CI 1.07 to 2.05; P=0.017). Treatment had a statistically significant effect on readiness to change use at 12 months (adjusted OR 2.05, 95% CI 1.26 to 3.31; P=0.004) that was not maintained at 24 months (0.78, 95% CI 0.48 to 1.28; P=0.320). There were no effects of treatment on clinical outcomes such as relapses, psychotic symptoms, functioning, and self harm.
Conclusions: Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and substance misuse do not improve outcome in terms of hospitalisation, symptom outcomes, or functioning. This approach does reduce the amount of substance used for at least one year after completion of therapy.
Trial registration: Current Controlled Trials: ISRCTN14404480.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures

Comment in
-
Psychosis and comorbid substance misuse: integrated motivational interviewing and cognitive behavioural therapy reduces alcohol intake.Evid Based Ment Health. 2011 May;14(2):51. doi: 10.1136/ebmh.14.2.51. Evid Based Ment Health. 2011. PMID: 21502157 No abstract available.
References
-
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264:2511-8. - PubMed
-
- Graham HL, Copello A, Birchwood MJ, Maslin J, McGovern D, Orford J, et al. The combined psychosis and substance use (COMPASS) programme: an integrated, shared care approach. In: Graham HL, Copello A, Birchwood MJ, Mueser KT, eds. Substance misuse in psychosis: approaches to treatment and service delivery. John Wiley & Sons, 2003:106-20.
-
- Weaver T, Rutter D, Madden P, Ward J, Stimson G, Renton A. Results of a screening survey for co-morbid substance misuse amongst patients in treatment for psychotic disorders: prevalence and service needs in an inner London borough. Soc Psychiatry Psychiatr Epidemiol 2001;36:399-406. - PubMed
-
- Dixon L, Haas G, Weiden PJ, Sweeney J, Frances AJ. Drug abuse in schizophrenic patients: clinical correlates and reasons for use. Am J Psychiatry 1991;148:224-30. - PubMed
-
- Maslin J. Substance misuse in psychosis: contextual issues. In: Graham HL, Copello A, Birchwood MJ, Mueser KT, eds. Substance misuse in psychosis: approaches to treatment and service delivery. John Wiley & Sons, 2003:3-23.
