Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells
- PMID: 21106690
- PMCID: PMC3074626
- DOI: 10.1152/ajpcell.00318.2010
Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells
Abstract
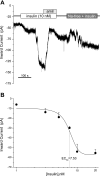
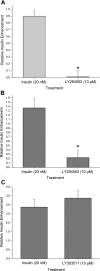
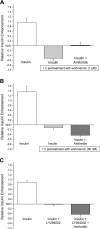
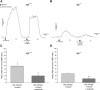
Diabetes is a profound disease that results in a severe lack of regulation of systemic salt and water balance. From our earlier work on the endocrine regulation of salt taste at the level of the epithelial sodium channel (ENaC), we have begun to investigate the ability of insulin to alter ENaC function with patch-clamp recording on isolated mouse taste receptor cells (TRCs). In fungiform and vallate TRCs that exhibit functional ENaC currents (e.g., amiloride-sensitive Na(+) influx), insulin (5-20 nM) caused a significant increase in Na(+) influx at -80 mV (EC(50) = 7.53 nM). The insulin-enhanced currents were inhibited by amiloride (30 μM). Similarly, in ratiometric Na(+) imaging using SBFI, insulin treatment (20 nM) enhanced Na(+) movement in TRCs, consistent with its action in electrophysiological assays. The ability of insulin to regulate ENaC function is dependent on the enzyme phosphoinositide 3-kinase since treatment with the inhibitor LY294002 (10 μM) abolished insulin-induced changes in ENaC. To test the role of insulin in the regulation of salt taste, we have characterized behavioral responses to NaCl using a mouse model of acute hyperinsulinemia. Insulin-treated mice show significant avoidance of NaCl at lower concentrations than the control group. Interestingly, these differences between groups were abolished when amiloride (100 μM) was added into NaCl solutions, suggesting that insulin was regulating ENaC. Our results are consistent with a role for insulin in maintaining functional expression of ENaC in mouse TRCs.
Figures



References
-
- Balla T. Pharmacology of phosphoinositides, regulators of multiple cellular functions. Curr Pharm Des 7: 475–507, 2001 - PubMed
-
- Blazer-Yost BL, Vahle JC, Byars JM, Bacallao RL. Real-time three-dimensional imaging of lipid signal transduction: apical membrane insertion of epithelial Na+ channels. Am J Physiol Cell Physiol 287: C1569–C1576, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

