Decreased hepatic breast cancer resistance protein expression and function in multidrug resistance-associated protein 2-deficient (TR⁻) rats
- PMID: 21106720
- PMCID: PMC3061562
- DOI: 10.1124/dmd.110.035188
Decreased hepatic breast cancer resistance protein expression and function in multidrug resistance-associated protein 2-deficient (TR⁻) rats
Abstract
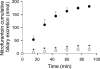
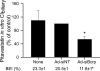
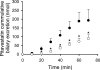
Multidrug resistance-associated protein (Mrp) 2-deficient (TR(-)) Wistar rats have been used to elucidate the role of Mrp2 in drug disposition. Decreased breast cancer resistance protein (Bcrp) levels were reported in sandwich-cultured hepatocytes (SCH) from TR(-) rats compared with those from wild-type (WT) rats. This study was designed to characterize hepatic Bcrp expression and function in TR(-) rats, using nitrofurantoin and pitavastatin as substrates. Bcrp was knocked down by RNA interference in rat SCH. Antibody BXP53, but not BXP21, specifically detected Bcrp knockdown in SCH. Bcrp protein levels were decreased markedly in TR(-) but not Mrp2-deficient Sprague-Dawley [Eisai hyperbilirubinemic rats (EHBR)] rats. Bcrp mRNA levels were decreased significantly in TR(-) livers as determined by TaqMan real-time reverse transcriptase-polymerase chain reaction. Biliary excretion of nitrofurantoin, a specific Bcrp substrate, was decreased significantly in SCH and isolated perfused livers from TR(-) rats compared with those from WT controls, indicating that hepatic Bcrp function is decreased in TR(-) rats. In Bcrp knockdown SCH, the biliary excretion index and in vitro biliary clearance of pitavastatin were decreased significantly to ∼ 58 and ∼ 52% of control, respectively, indicating that Bcrp plays a role in pitavastatin biliary excretion. Pitavastatin biliary excretion was decreased significantly in perfused livers from TR(-) compared with those from WT rats. In conclusion, expression and function of hepatic Bcrp are decreased significantly in TR(-) rats. The potential role of both Bcrp and Mrp2 should be considered when data generated in TR(-) rats are interpreted. TR(-) and EHBR rats in combination may be useful in differentiating the role of Mrp2 and Bcrp in drug/metabolite disposition.
Figures
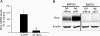


Similar articles
-
An experimental approach to evaluate the impact of impaired transport function on hepatobiliary drug disposition using Mrp2-deficient TR- rat sandwich-cultured hepatocytes in combination with Bcrp knockdown.Mol Pharm. 2014 Mar 3;11(3):766-75. doi: 10.1021/mp400471e. Epub 2014 Jan 30. Mol Pharm. 2014. PMID: 24410402 Free PMC article.
-
Knocking down breast cancer resistance protein (Bcrp) by adenoviral vector-mediated RNA interference (RNAi) in sandwich-cultured rat hepatocytes: a novel tool to assess the contribution of Bcrp to drug biliary excretion.Mol Pharm. 2009 Jan-Feb;6(1):134-43. doi: 10.1021/mp800100e. Mol Pharm. 2009. PMID: 19105722 Free PMC article.
-
Hepatic basolateral efflux contributes significantly to rosuvastatin disposition II: characterization of hepatic elimination by basolateral, biliary, and metabolic clearance pathways in rat isolated perfused liver.J Pharmacol Exp Ther. 2013 Dec;347(3):737-45. doi: 10.1124/jpet.113.208314. Epub 2013 Sep 30. J Pharmacol Exp Ther. 2013. PMID: 24080682 Free PMC article.
-
Hepatocellular exposure of troglitazone metabolites in rat sandwich-cultured hepatocytes lacking Bcrp and Mrp2: interplay between formation and excretion.Drug Metab Dispos. 2014 Jul;42(7):1219-26. doi: 10.1124/dmd.114.057190. Epub 2014 May 5. Drug Metab Dispos. 2014. PMID: 24799397 Free PMC article.
-
[Frontline in Biliary Excretion of Drugs and Their Evaluation Systems].Yakugaku Zasshi. 2025;145(6):491-499. doi: 10.1248/yakushi.24-00165-1. Yakugaku Zasshi. 2025. PMID: 40451870 Review. Japanese.
Cited by
-
Characterization of Liver- and Cancer-type-Organic Anion Transporting Polypeptide (OATP) 1B3 Messenger RNA Expression in Normal and Cancerous Human Tissues.Drug Metab Lett. 2018;12(1):24-32. doi: 10.2174/1872312812666180326110146. Drug Metab Lett. 2018. PMID: 29577869 Free PMC article.
-
Regulation of hepatic P-gp expression and activity by genistein in rats.Arch Toxicol. 2020 May;94(5):1625-1635. doi: 10.1007/s00204-020-02708-3. Epub 2020 Mar 18. Arch Toxicol. 2020. PMID: 32185415
-
Endogenous Coproporphyrin I and III are Altered in Multidrug Resistance-Associated Protein 2-Deficient (TR-) Rats.J Pharm Sci. 2021 Jan;110(1):404-411. doi: 10.1016/j.xphs.2020.10.017. Epub 2020 Oct 13. J Pharm Sci. 2021. PMID: 33058892 Free PMC article.
-
Placental and fetal disposition of mercuric ions in rats exposed to methylmercury: role of Mrp2.Reprod Toxicol. 2012 Dec;34(4):628-34. doi: 10.1016/j.reprotox.2012.10.001. Epub 2012 Oct 8. Reprod Toxicol. 2012. PMID: 23059061 Free PMC article.
-
Pitavastatin suppressed liver cancer cells in vitro and in vivo.Onco Targets Ther. 2016 Aug 29;9:5383-8. doi: 10.2147/OTT.S106906. eCollection 2016. Onco Targets Ther. 2016. PMID: 27621652 Free PMC article.
References
-
- Abe K, Bridges AS, Yue W, Brouwer KLR. (2008) In vitro biliary clearance of angiotensin II receptor blockers and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in sandwich-cultured rat hepatocytes: comparison with in vivo biliary clearance. J Pharmacol Exp Ther 326:983–990 - PMC - PubMed
-
- Chandra P, Brouwer KLR. (2004) The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res 21:719–735 - PubMed
-
- Choudhuri S, Klaassen CD. (2006) Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol 25:231–259 - PubMed
-
- Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, et al. (2006) Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2). J Pharmacol Exp Ther 317:579–589 - PubMed
-
- Dietrich CG, de Waart DR, Ottenhoff R, Bootsma AH, van Gennip AH, Elferink RP. (2001) Mrp2-deficiency in the rat impairs biliary and intestinal excretion and influences metabolism and disposition of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis 22:805–811 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

