HIV-1 maturation inhibitor bevirimat stabilizes the immature Gag lattice
- PMID: 21106735
- PMCID: PMC3028903
- DOI: 10.1128/JVI.01926-10
HIV-1 maturation inhibitor bevirimat stabilizes the immature Gag lattice
Abstract
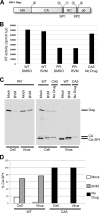
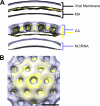
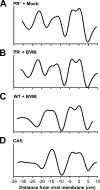
Maturation of nascent virions, a key step in retroviral replication, involves cleavage of the Gag polyprotein by the viral protease into its matrix (MA), capsid (CA), and nucleocapsid (NC) components and their subsequent reorganization. Bevirimat (BVM) defines a new class of antiviral drugs termed maturation inhibitors. BVM acts by blocking the final cleavage event in Gag processing, the separation of CA from its C-terminal spacer peptide 1 (SP1). Prior evidence suggests that BVM binds to Gag assembled in immature virions, preventing the protease from accessing the CA-SP1 cleavage site. To investigate this hypothesis, we used cryo-electron tomography to examine the structures of (noninfectious) HIV-1 viral particles isolated from BVM-treated cells. We find that these particles contain an incomplete shell of density underlying the viral envelope, with a hexagonal honeycomb structure similar to the Gag lattice of immature HIV but lacking the innermost, NC-related, layer. We conclude that the shell represents a remnant of the immature Gag lattice that has been processed, except at the CA-SP1 sites, but has remained largely intact. We also compared BVM-treated particles with virions formed by the mutant CA5, in which cleavage between CA and SP1 is also blocked. Here, we find a thinner CA-related shell with no visible evidence of honeycomb organization, indicative of an altered conformation and further suggesting that binding of BVM stabilizes the immature lattice. In both cases, the observed failure to assemble mature capsids correlates with the loss of infectivity.
Figures



References
-
- Adamson, C. S., and E. O. Freed. 2007. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv. Pharmacol. 55:347-387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

