Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice
- PMID: 21106754
- PMCID: PMC3003105
- DOI: 10.1073/pnas.1016233107
Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice
Abstract
Permanent scars form upon healing of tissue injuries such as those caused by ischemia (myocardial infarction, stroke), trauma, surgery, and inflammation. Current options in reducing scar formation are limited to local intervention. We have designed a systemically administered, target-seeking biotherapeutic for scar prevention. It consists of a vascular targeting peptide that specifically recognizes angiogenic blood vessels and extravasates into sites of injury, fused with a therapeutic molecule, decorin. Decorin prevents tissue fibrosis and promotes tissue regeneration by inhibiting TGF-β activity and by other regulatory activities. The decorin-targeting peptide fusion protein had substantially increased neutralizing activity against TGF-β1 in vitro compared with untargeted decorin. In vivo, the fusion protein selectively accumulated in wounds, and promoted wound healing and suppressed scar formation at doses where nontargeted decorin was inactive. These results show that selective targeting yields a tissue-healing and scar-reducing compound with enhanced specificity and potency. This approach may help make reducing scar formation by systemic drug delivery a feasible option for surgery and for the treatment of pathological processes in which scar formation is a problem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

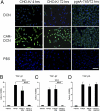
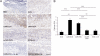
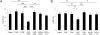
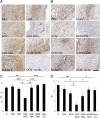
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

