Neural representations of relevant and irrelevant features in perceptual decision making
- PMID: 21106817
- PMCID: PMC3020592
- DOI: 10.1523/JNEUROSCI.3163-10.2010
Neural representations of relevant and irrelevant features in perceptual decision making
Abstract
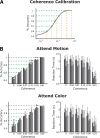
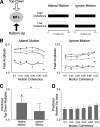
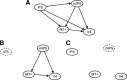
Although perceptual decision making activates a network of brain areas involved in sensory, integrative, and motor functions, circuit activity can clearly be modulated by factors beyond the stimulus. Of particular interest is to understand how the network is modulated by top-down factors such as attention. Here, we demonstrate in a motion coherence task that selective attention produces marked changes in the blood oxygen level-dependent (BOLD) response in a subset of regions within a human perceptual decision-making circuit. Specifically, when motion is attended, the BOLD response decreases with increasing motion coherence in many regions, including the motion-sensitive area MT+, the intraparietal sulcus, and the inferior frontal sulcus. However, when motion is ignored, the negative parametric response in a subset of this circuit becomes positive. Through both modeling and connectivity analyses, we demonstrate that this inversion both reflects a top-down influence and segregates attentional from accumulation regions, thereby permitting us to further delineate the contributions of different regions to the perceptual decision.
Figures



References
-
- Aron AR, Robbins TW, Poldrack RA. Inhibition the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. - PubMed
-
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward BD. Neural correlates of sensory and decision processes in auditory object identification. Nat Neurosci. 2004;7:295–301. - PubMed
-
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
