Orchestration of stepwise synaptic growth by K+ and Ca2+ channels in Drosophila
- PMID: 21106821
- PMCID: PMC3075884
- DOI: 10.1523/JNEUROSCI.3448-10.2010
Orchestration of stepwise synaptic growth by K+ and Ca2+ channels in Drosophila
Abstract
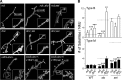
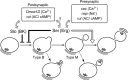
Synapse formation is tightly associated with neuronal excitability. We found striking synaptic overgrowth caused by Drosophila K(+)-channel mutations of the seizure and slowpoke genes, encoding Erg and Ca(2+)-activated large-conductance (BK) channels, respectively. These mutants display two distinct patterns of "satellite" budding from larval motor terminus synaptic boutons. Double-mutant analysis indicates that BK and Erg K(+) channels interact with separate sets of synaptic proteins to affect distinct growth steps. Post-synaptic L-type Ca(2+) channels, Dmca1D, and PSD-95-like scaffold protein, Discs large, are required for satellite budding induced by slowpoke and seizure mutations. Pre-synaptic cacophony Ca(2+) channels and the NCAM-like adhesion molecule, Fasciclin II, take part in a maturation step that is partially arrested by seizure mutations. Importantly, slowpoke and seizure satellites were both suppressed by rutabaga mutations that disrupt Ca(2+)/CaM-dependent adenylyl cyclase, demonstrating a convergence of K(+) channels of different functional categories in regulation of excitability-dependent Ca(2+) influx for triggering cAMP-mediated growth plasticity.
Figures

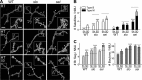
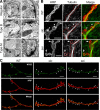
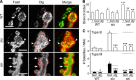
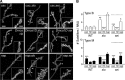
Similar articles
-
Distinct roles of Drosophila cacophony and Dmca1D Ca(2+) channels in synaptic homeostasis: genetic interactions with slowpoke Ca(2+) -activated BK channels in presynaptic excitability and postsynaptic response.Dev Neurobiol. 2014 Jan;74(1):1-15. doi: 10.1002/dneu.22120. Epub 2013 Oct 7. Dev Neurobiol. 2014. PMID: 23959639 Free PMC article.
-
Dendritic and Axonal L-Type Calcium Channels Cooperate to Enhance Motoneuron Firing Output during Drosophila Larval Locomotion.J Neurosci. 2017 Nov 8;37(45):10971-10982. doi: 10.1523/JNEUROSCI.1064-17.2017. Epub 2017 Oct 6. J Neurosci. 2017. PMID: 28986465 Free PMC article.
-
Sub-cellular Ca2+ dynamics affected by voltage- and Ca2+-gated K+ channels: Regulation of the soma-growth cone disparity and the quiescent state in Drosophila neurons.Neuroscience. 2006 Oct 27;142(3):629-44. doi: 10.1016/j.neuroscience.2006.06.051. Epub 2006 Aug 17. Neuroscience. 2006. PMID: 16919393
-
Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage.Biol Res. 2006;39(3):385-401. doi: 10.4067/s0716-97602006000300003. Epub 2006 Nov 7. Biol Res. 2006. PMID: 17106573 Review.
-
Behavioral and electrophysiological analysis of Ca-activated K-channel transgenes in Drosophila.Ann N Y Acad Sci. 1998 Nov 16;860:296-305. doi: 10.1111/j.1749-6632.1998.tb09057.x. Ann N Y Acad Sci. 1998. PMID: 9928320 Review.
Cited by
-
Development and plasticity of the Drosophila larval neuromuscular junction.Wiley Interdiscip Rev Dev Biol. 2013 Sep-Oct;2(5):647-70. doi: 10.1002/wdev.108. Epub 2013 Feb 5. Wiley Interdiscip Rev Dev Biol. 2013. PMID: 24014452 Free PMC article. Review.
-
Transmission, Development, and Plasticity of Synapses.Genetics. 2015 Oct;201(2):345-75. doi: 10.1534/genetics.115.176529. Genetics. 2015. PMID: 26447126 Free PMC article. Review.
-
Brain tumor regulates neuromuscular synapse growth and endocytosis in Drosophila by suppressing mad expression.J Neurosci. 2013 Jul 24;33(30):12352-63. doi: 10.1523/JNEUROSCI.0386-13.2013. J Neurosci. 2013. PMID: 23884941 Free PMC article.
-
The Drosophila ERG channel seizure plays a role in the neuronal homeostatic stress response.PLoS Genet. 2019 Aug 8;15(8):e1008288. doi: 10.1371/journal.pgen.1008288. eCollection 2019 Aug. PLoS Genet. 2019. PMID: 31393878 Free PMC article.
-
MAPK3 at the Autism-Linked Human 16p11.2 Locus Influences Precise Synaptic Target Selection at Drosophila Larval Neuromuscular Junctions.Mol Cells. 2017 Feb;40(2):151-161. doi: 10.14348/molcells.2017.2307. Epub 2017 Feb 15. Mol Cells. 2017. PMID: 28196412 Free PMC article.
References
-
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
-
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol. 1993;24:1008–1024. - PubMed
-
- Bailey CH, Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983;220:91–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
