Calcium regulates vesicle replenishment at the cone ribbon synapse
- PMID: 21106825
- PMCID: PMC3018691
- DOI: 10.1523/JNEUROSCI.2891-10.2010
Calcium regulates vesicle replenishment at the cone ribbon synapse
Abstract
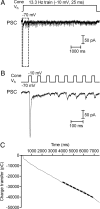
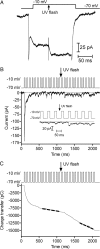
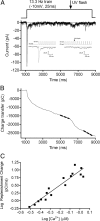
Cones release glutamate-filled vesicles continuously in darkness, and changing illumination modulates this release. Because sustained release in darkness is governed by vesicle replenishment rates, we analyzed how cone membrane potential regulates replenishment. Synaptic release from cones was measured by recording postsynaptic currents in Ambystoma tigrinum horizontal or OFF bipolar cells evoked by depolarization of simultaneously voltage-clamped cones. We measured replenishment after attaining a steady state between vesicle release and replenishment using trains of test pulses. Increasing Ca(2+) currents (I(Ca)) by changing the test step from -30 to -10 mV increased replenishment. Lengthening -30 mV test pulses to match the Ca(2+) influx during 25 ms test pulses to -10 mV produced similar replenishment rates. Reducing Ca(2+) driving force by using test steps to +30 mV slowed replenishment. Using UV flashes to reverse inhibition of I(Ca) by nifedipine accelerated replenishment. Increasing [Ca(2+)](i) by flash photolysis of caged Ca(2+) also accelerated replenishment. Replenishment, but not the initial burst of release, was enhanced by using an intracellular Ca(2+) buffer of 0.5 mm EGTA rather than 5 mm EGTA, and diminished by 1 mm BAPTA. This suggests that although release and replenishment exhibited similar Ca(2+) dependencies, release sites are <200 nm from Ca(2+) channels but replenishment sites are >200 nm away. Membrane potential thus regulates replenishment by controlling Ca(2+) influx, principally by effects on replenishment mechanisms but also by altering releasable pool size. This in turn provides a mechanism for converting changes in light intensity into changes in sustained release at the cone ribbon synapse.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
