Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus
- PMID: 21106841
- PMCID: PMC3073577
- DOI: 10.1523/JNEUROSCI.3898-10.2010
Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus
Abstract
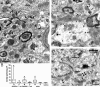
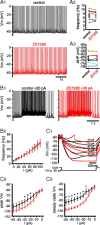
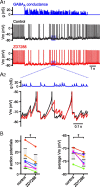
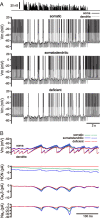
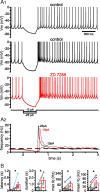
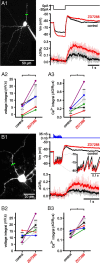
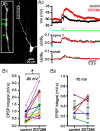
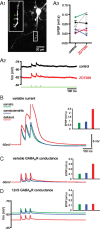
The activity patterns of subthalamic nucleus (STN) neurons are intimately linked to motor function and dysfunction and arise through the complex interaction of intrinsic properties and inhibitory and excitatory synaptic inputs. In many neurons, hyperpolarization-activated cyclic nucleotide-gated (HCN) channels play key roles in intrinsic excitability and synaptic integration both under normal conditions and in disease states. However, in STN neurons, which strongly express HCN channels, their roles remain relatively obscure. To address this deficit, complementary molecular and cellular electrophysiological, imaging, and computational approaches were applied to the rat STN. Molecular profiling demonstrated that individual STN neurons express mRNA encoding several HCN subunits, with HCN2 and 3 being the most abundant. Light and electron microscopic analysis showed that HCN2 subunits are strongly expressed and distributed throughout the somatodendritic plasma membrane. Voltage-, current-, and dynamic-clamp analysis, two-photon Ca(2+) imaging, and computational modeling revealed that HCN channels are activated by GABA(A) receptor-mediated inputs and thus limit synaptic hyperpolarization and deinactivation of low-voltage-activated Ca(2+) channels. Although HCN channels also limited the temporal summation of EPSPs, generated through two-photon uncaging of glutamate, this action was largely shunted by GABAergic inhibition that was necessary for HCN channel activation. Together the data demonstrate that HCN channels in STN neurons selectively counteract GABA(A) receptor-mediated inhibition arising from the globus pallidus and thus promote single-spike activity rather than rebound burst firing.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
