Adenosine A2A receptor activation attenuates tubuloglomerular feedback responses by stimulation of endothelial nitric oxide synthase
- PMID: 21106859
- PMCID: PMC3044007
- DOI: 10.1152/ajprenal.00567.2010
Adenosine A2A receptor activation attenuates tubuloglomerular feedback responses by stimulation of endothelial nitric oxide synthase
Abstract
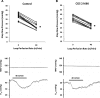
Adenosine A(2) receptors have been suggested to modulate tubuloglomerular feedback (TGF) responses by counteracting adenosine A(1) receptor-mediated vasoconstriction, but the mechanisms are unclear. We tested the hypothesis that A(2A) receptor activation blunts TGF by release of nitric oxide in the juxtaglomerular apparatus (JGA). Maximal TGF responses were measured in male Sprague-Dawley rats as changes in proximal stop-flow pressure (ΔP(SF)) in response to increased perfusion of the loop of Henle (0 to 40 nl/min) with artificial tubular fluid (ATF). The maximal TGF response was studied after 5 min intratubular perfusion (10 nl/min) with ATF or ATF + A(2A) receptor agonist (CGS-21680; 10(-7) mol/l). The interaction with nitric oxide synthase (NOS) isoforms was tested by perfusion with a nonselective NOS inhibitor [N(ω)-nitro-L-arginine methyl ester hydrochloride (L-NAME); 10(-3) mol/l] or a selective neuronal NOS (nNOS) inhibitor [N(ω)-propyl-L-arginine (L-NPA); 10(-6) mol/l] alone, and with the A(2A) agonist. Blood pressure, urine flow, and P(SF) at 0 nl/min were similar among the groups. The maximal TGF response (ΔP(SF)) with ATF alone (12.3 ± 0.6 mmHg) was attenuated by selective A(2A) stimulation (9.5 ± 0.4 mmHg). L-NAME enhanced maximal TGF responses (18.9 ± 0.4 mmHg) significantly more than L-NPA (15.2 ± 0.7 mmHg). Stimulation of A(2A) receptors did not influence maximal TGF response during nonselective NOS inhibition (19.0 ± 0.4) but attenuated responses during nNOS inhibition (10.3 ± 0.4 mmHg). In conclusion, adenosine A(2A) receptor activation attenuated TGF responses by stimulation of endothelial NOS (eNOS), presumably in the afferent arteriole. Moreover, NO derived from both eNOS and nNOS in the JGA may blunt TGF responses.
Figures



Similar articles
-
Adenosine A(2) receptors modulate tubuloglomerular feedback.Am J Physiol Renal Physiol. 2010 Aug;299(2):F412-7. doi: 10.1152/ajprenal.00211.2010. Epub 2010 Jun 2. Am J Physiol Renal Physiol. 2010. PMID: 20519378 Free PMC article.
-
Neuronal nitric oxide synthase inhibition sensitizes the tubuloglomerular feedback mechanism after volume expansion.Kidney Int. 2004 Apr;65(4):1349-56. doi: 10.1111/j.1523-1755.2004.00509.x. Kidney Int. 2004. PMID: 15086474
-
Cyclooxygenase 2 inhibition suppresses tubuloglomerular feedback: roles of thromboxane receptors and nitric oxide.Am J Physiol Renal Physiol. 2009 Apr;296(4):F790-4. doi: 10.1152/ajprenal.90446.2008. Epub 2009 Jan 14. Am J Physiol Renal Physiol. 2009. PMID: 19144694 Free PMC article.
-
Nitric oxide and tubuloglomerular feedback.Semin Nephrol. 1999 May;19(3):251-62. Semin Nephrol. 1999. PMID: 10226331 Review.
-
Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice.Kidney Int. 2008 Aug;74(4):418-26. doi: 10.1038/ki.2008.145. Epub 2008 Apr 16. Kidney Int. 2008. PMID: 18418352 Free PMC article. Review.
Cited by
-
Adenosine A2A receptor antagonists act at the hyperoxic phase to confer protection against retinopathy.Mol Med. 2018 Jul 31;24(1):41. doi: 10.1186/s10020-018-0038-1. Mol Med. 2018. PMID: 30134834 Free PMC article.
-
Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors.Am J Physiol Renal Physiol. 2012 Oct 15;303(8):F1166-75. doi: 10.1152/ajprenal.00222.2012. Epub 2012 Aug 15. Am J Physiol Renal Physiol. 2012. PMID: 22896040 Free PMC article.
-
Effect of nitric oxide on renal autoregulation during hypothermia in the rat.Pflugers Arch. 2017 Jun;469(5-6):669-680. doi: 10.1007/s00424-017-1967-1. Epub 2017 Mar 17. Pflugers Arch. 2017. PMID: 28315005 Free PMC article.
-
Genetic Abrogation of Adenosine A3 Receptor Prevents Uninephrectomy and High Salt-Induced Hypertension.J Am Heart Assoc. 2016 Jul 18;5(7):e003868. doi: 10.1161/JAHA.116.003868. J Am Heart Assoc. 2016. PMID: 27431647 Free PMC article.
-
Identification and function of adenosine A3 receptor in afferent arterioles.Am J Physiol Renal Physiol. 2015 May 1;308(9):F1020-5. doi: 10.1152/ajprenal.00422.2014. Epub 2015 Jan 21. Am J Physiol Renal Physiol. 2015. PMID: 25608966 Free PMC article.
References
-
- Al-Mashhadi RH, Skott O, Vanhoutte PM, Hansen PB. Activation of A(2) adenosine receptors dilates cortical efferent arterioles in mouse. Kidney Int 75: 793–799, 2009 - PubMed
-
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001 - PubMed
-
- Brown R, Ollerstam A, Persson AE. Neuronal nitric oxide synthase inhibition sensitizes the tubuloglomerular feedback mechanism after volume expansion. Kidney Int 65: 1349–1356, 2004 - PubMed
-
- Carlstrom M, Lai EY, Ma Z, Patzak A, Brown RD, Persson AE. Role of NOX2 in the regulation of afferent arteriole responsiveness. Am J Physiol Regul Integr Comp Physiol 296: R72–R79, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

