Glucocorticoids differentially regulate the expression of CRFR1 and CRFR2α in MIN6 insulinoma cells and rodent islets
- PMID: 21106875
- PMCID: PMC3219054
- DOI: 10.1210/en.2010-0791
Glucocorticoids differentially regulate the expression of CRFR1 and CRFR2α in MIN6 insulinoma cells and rodent islets
Abstract
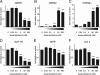
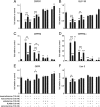
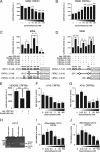
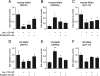
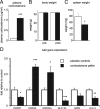
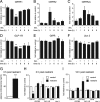
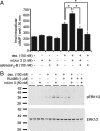
Urocortin 3 (Ucn 3), member of the corticotropin-releasing factor (CRF) family of peptide hormones, is released from β-cells to potentiate insulin secretion. Ucn 3 activates the CRF type-2 receptor (CRFR2) but does not activate the type-1 receptor (CRFR1), which was recently demonstrated on β-cells. While the direct actions of Ucn 3 on insulin secretion suggest the presence of cognate receptors within the islet microenvironment, this has not been established. Here we demonstrate that CRFR2α is expressed by MIN6 insulinoma cells and by primary mouse and human islets, with no detectable expression of CRFR2β. Furthermore, stimulation of MIN6 cells or primary mouse islets in vitro or in vivo with glucocorticoids (GCs) robustly and dose-dependently increases the expression of CRFR2α, while simultaneously inhibiting the expression of CRFR1 and incretin receptors. Luciferase reporters driven by the mouse CRFR1 or CRFR2α promoter in MIN6 cells confirm these differential effects of GCs. In contrast, GCs inhibit CRFR2α promoter activity in HEK293 cells and inhibit the expression of CRFR2β in A7r5 rat aortic smooth muscle cells and differentiated C2C12 myotubes. These findings suggest that the GC-mediated increase of CRFR2α depends on the cellular context of the islet and deviates from the GC-mediated suppression of CRFR1 and incretin receptors. Furthermore, GC-induced increases in CRFR2α expression coincide with increased Ucn 3-dependent activation of cAMP and MAPK pathways. We postulate that differential effect of GCs on the expression of CRFR1 and CRFR2α in the endocrine pancreas represent a mechanism to shift sensitivity from CRFR1 to CRFR2 ligands.
Figures

Similar articles
-
Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids.Mol Endocrinol. 2005 Feb;19(2):441-58. doi: 10.1210/me.2004-0300. Epub 2004 Oct 28. Mol Endocrinol. 2005. PMID: 15514029
-
Structural basis for hormone recognition by the Human CRFR2{alpha} G protein-coupled receptor.J Biol Chem. 2010 Dec 17;285(51):40351-61. doi: 10.1074/jbc.M110.186072. Epub 2010 Oct 21. J Biol Chem. 2010. PMID: 20966082 Free PMC article.
-
CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner.Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):912-7. doi: 10.1073/pnas.0913610107. Epub 2009 Dec 22. Proc Natl Acad Sci U S A. 2010. PMID: 20080775 Free PMC article.
-
Corticotropin-releasing factor family and its receptors: tumor therapeutic targets?Biochem Biophys Res Commun. 2007 Nov 3;362(4):785-8. doi: 10.1016/j.bbrc.2007.08.014. Epub 2007 Aug 29. Biochem Biophys Res Commun. 2007. PMID: 17822675 Review.
-
The CRF Family of Neuropeptides and their Receptors - Mediators of the Central Stress Response.Curr Mol Pharmacol. 2018;11(1):4-31. doi: 10.2174/1874467210666170302104053. Curr Mol Pharmacol. 2018. PMID: 28260504 Free PMC article. Review.
Cited by
-
Maturation of stem cell-derived beta-cells guided by the expression of urocortin 3.Rev Diabet Stud. 2014 Spring;11(1):115-32. doi: 10.1900/RDS.2014.11.115. Epub 2014 May 10. Rev Diabet Stud. 2014. PMID: 25148370 Free PMC article. Review.
-
Artemether Does Not Turn α Cells into β Cells.Cell Metab. 2018 Jan 9;27(1):218-225.e4. doi: 10.1016/j.cmet.2017.10.002. Epub 2017 Nov 2. Cell Metab. 2018. PMID: 29103923 Free PMC article.
-
Urocortin Neuropeptide Levels Are Impaired in the PBMCs of Overweight Children.Nutrients. 2022 Jan 18;14(3):429. doi: 10.3390/nu14030429. Nutrients. 2022. PMID: 35276788 Free PMC article.
-
The Difference δ-Cells Make in Glucose Control.Physiology (Bethesda). 2018 Nov 1;33(6):403-411. doi: 10.1152/physiol.00029.2018. Physiology (Bethesda). 2018. PMID: 30303773 Free PMC article. Review.
-
Research on the Effects of the Chronic Treatment With Different Doses of Urocortin 2 in Heart Failure Rats.Dose Response. 2019 Jun 26;17(2):1559325819860018. doi: 10.1177/1559325819860018. eCollection 2019 Apr-Jun. Dose Response. 2019. PMID: 31263386 Free PMC article.
References
-
- Strowski MZ, Parmar RM, Blake AD, Schaeffer JM 2000 Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141:111–117 - PubMed
-
- Baggio LL, Drucker DJ 2007 Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 - PubMed
-
- Hansotia T, Drucker DJ 2005 GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept 128:125–134 - PubMed
-
- Gilon P, Henquin JC 2001 Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 22:565–604 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

