The prototypical ranitidine analog JWS-USC-75-IX improves information processing and cognitive function in animal models
- PMID: 21106907
- PMCID: PMC3061527
- DOI: 10.1124/jpet.110.175422
The prototypical ranitidine analog JWS-USC-75-IX improves information processing and cognitive function in animal models
Abstract
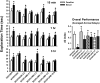
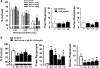
This study was designed to evaluate further a prototypical ranitidine analog, JWS-USC-75-IX, [(3-[[[2-[[(5-dimethylaminomethyl)-2-furanyl]methyl]thio]ethyl]amino]-4-nitropyridazine, JWS], for neuropharmacologic properties that would theoretically be useful for treating cognitive and noncognitive behavioral symptoms of neuropsychiatric disorders. JWS was previously found to inhibit acetylcholinesterase (AChE) activity, serve as a potent ligand at muscarinic M₂ acetylcholine receptors, and elicit positive effects on spatial learning, passive avoidance, and working memory in rodents. In the current study, JWS was evaluated for binding activity at more than 60 neurotransmitter receptors, transporters, and ion channels, as well as for inhibitory activity at AChE and butyrylcholinesterase (BChE). The results indicate that JWS inhibits AChE and BChE at low (micromolar) concentrations and that it is a functional antagonist at M₂ receptors (K(B) = 320 nM). JWS was subsequently evaluated orally across additional behavioral assays in rodents (dose range, 0.03-10.0 mg/kg) as well as nonhuman primates (dose range, 0.05-2.0 mg/kg). In rats, JWS improved prepulse inhibition (PPI) of the acoustic startle response in nonimpaired rats and attenuated PPI deficits in three pharmacologic impairment models. JWS also attenuated scopolamine and (-)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801)-related impairments in a spontaneous novel object recognition task and a five-choice serial reaction time task, respectively. In monkeys, JWS elicited dose-dependent improvements of a delayed match-to-sample task as well as an attention-related version of the task where randomly presented (task-relevant) distractors were presented. Thus, JWS (potentially via effects at several drug targets) improves information processing, attention, and memory in animal models and could potentially treat the cognitive and behavioral symptoms of some neuropsychiatric illnesses.
Figures


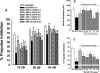
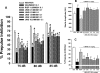


Similar articles
-
Synthesis and biological evaluation of ranitidine analogs as multiple-target-directed cognitive enhancers for the treatment of Alzheimer's disease.Bioorg Med Chem Lett. 2016 Nov 15;26(22):5573-5579. doi: 10.1016/j.bmcl.2016.09.072. Epub 2016 Oct 6. Bioorg Med Chem Lett. 2016. PMID: 27769620 Free PMC article.
-
Acute NMDA receptor antagonism impairs working memory performance but not attention in rats-Implications for the NMDAr hypofunction theory of schizophrenia.Behav Neurosci. 2020 Aug;134(4):323-331. doi: 10.1037/bne0000402. Behav Neurosci. 2020. PMID: 32658524
-
SIB-1553A, (+/-)-4-[[2-(1-methyl-2-pyrrolidinyl)ethyl]thio]phenol hydrochloride, a subtype-selective ligand for nicotinic acetylcholine receptors with putative cognitive-enhancing properties: effects on working and reference memory performances in aged rodents and nonhuman primates.J Pharmacol Exp Ther. 2001 Oct;299(1):297-306. J Pharmacol Exp Ther. 2001. PMID: 11561092
-
Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders.J Pharmacol Exp Ther. 2009 May;329(2):459-68. doi: 10.1124/jpet.108.150094. Epub 2009 Feb 17. J Pharmacol Exp Ther. 2009. PMID: 19223665
-
Ranitidine.2024 Sep 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2024 Sep 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 30000311 Free Books & Documents. Review.
Cited by
-
Use of Push-Pull Superfusion Technique for Identifying Neurotransmitters Involved in Brain Functions: Achievements and Perspectives.Curr Neuropharmacol. 2015;13(6):819-29. doi: 10.2174/1570159x13666150722233149. Curr Neuropharmacol. 2015. PMID: 26630960 Free PMC article. Review.
-
Neurobiology of nAChRs and cognition: a mini review of Dr. Jerry J. Buccafusco's contributions over a 25 year career.Biochem Pharmacol. 2011 Oct 15;82(8):883-90. doi: 10.1016/j.bcp.2011.06.010. Epub 2011 Jun 12. Biochem Pharmacol. 2011. PMID: 21684265 Free PMC article. Review.
-
Synthesis and biological evaluation of ranitidine analogs as multiple-target-directed cognitive enhancers for the treatment of Alzheimer's disease.Bioorg Med Chem Lett. 2016 Nov 15;26(22):5573-5579. doi: 10.1016/j.bmcl.2016.09.072. Epub 2016 Oct 6. Bioorg Med Chem Lett. 2016. PMID: 27769620 Free PMC article.
-
The prominent role of stimulus processing: cholinergic function and dysfunction in cognition.Curr Opin Neurol. 2011 Aug;24(4):364-70. doi: 10.1097/WCO.0b013e328348bda5. Curr Opin Neurol. 2011. PMID: 21725241 Free PMC article.
-
CNTRICS final animal model task selection: control of attention.Neurosci Biobehav Rev. 2013 Nov;37(9 Pt B):2099-110. doi: 10.1016/j.neubiorev.2012.05.009. Epub 2012 Jun 6. Neurosci Biobehav Rev. 2013. PMID: 22683929 Free PMC article. Review.
References
-
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, et al. (1998) Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 24:189–202 - PubMed
-
- Akhtar M, Uma Devi P, Ali A, Pillai KK, Vohora D. (2006) Antipsychotic-like profile of thioperamide, a selective H3-receptor antagonist in mice. Fundam Clin Pharmacol 20:373–378 - PubMed
-
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, Gill R, Juszczak E, Yu LM, Jacoby R, et al. (2009) The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol 8:151–157 - PubMed
-
- Ballmaier M, Casamenti F, Scali C, Mazzoncini R, Zoli M, Pepeu G, Spano PF. (2002) Rivastigmine antagonizes deficits in prepulse inhibition induced by selective immunolesioning of cholinergic neurons in nucleus basalis magnocellularis. Neuroscience 114:91–98 - PubMed
-
- Bürki HR. (1979) Extrapyramidal side-effects. Pharmacol Ther B 5:525–534 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

