A methyl-deficient diet fed to rat dams during the peri-conception period programs glucose homeostasis in adult male but not female offspring
- PMID: 21106931
- PMCID: PMC3001237
- DOI: 10.3945/jn.109.119453
A methyl-deficient diet fed to rat dams during the peri-conception period programs glucose homeostasis in adult male but not female offspring
Abstract
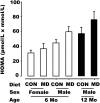
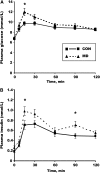
Methyl deficiencies have been implicated in metabolic programming during the periods of oocyte and embryo development. Semisynthetic methyl-deficient diets (MD) with no folic acid, 0.05% choline, and approximately one-half the recommended content of methionine were fed to female rats for 3 wk prior to mating and for the first 5 d of gestation. During the period of MD feeding, plasma homocysteine concentrations were approximately twice those of rats fed the complete (CON) diet. From d 5, both groups received a complete semipurified AIN diet until birth. On d 8, plasma homocysteine concentrations did not differ between the 2 groups. Thereafter, dams and offspring were fed a nonpurified diet for the remainder of the experiment. At 6 mo of age, the homeostatic model assessment (HOMA) index of the male MD offspring tended to be 32% higher (P = 0.053) and peak insulin during an oral glucose tolerance test (oGTT) was 39% higher (P < 0.05) compared with the male CON offspring. There was no difference in the response to an oGTT in the female offspring at 6 mo of age. The increased HOMA index of male MD offspring persisted to 12 mo of age. The peak glucose concentration during oGTT was 23% higher (P < 0.05) in MD compared with the CON males despite 39% greater (P < 0.05) peak insulin concentrations. This study shows that in rats, a physiologically relevant methyl-deficient diet fed during the period of oocyte maturation and preimplantation development programs gender-specific changes in glucose handling by the offspring.
Conflict of interest statement
Author disclosures: C. A. Maloney, S. M. Hay, L. E. Young, K. D. Sinclair, and W. D. Rees, no conflicts of interest.
Figures
Similar articles
-
The effects of feeding rats diets deficient in folic acid and related methyl donors on the blood pressure and glucose tolerance of the offspring.Br J Nutr. 2009 May;101(9):1333-40. doi: 10.1017/S0007114508066798. Epub 2008 Sep 10. Br J Nutr. 2009. PMID: 18782463
-
Chronic maternal vitamin B12 restriction induced changes in body composition & glucose metabolism in the Wistar rat offspring are partly correctable by rehabilitation.PLoS One. 2014 Nov 14;9(11):e112991. doi: 10.1371/journal.pone.0112991. eCollection 2014. PLoS One. 2014. PMID: 25398136 Free PMC article.
-
Pre- and postnatal methyl deficiency in the rat differentially alters glucose homeostasis.J Nutrigenet Nutrigenomics. 2011;4(4):175-91. doi: 10.1159/000330227. Epub 2011 Aug 23. J Nutrigenet Nutrigenomics. 2011. PMID: 21860247
-
Soya protein- and casein-based nutritionally complete diets fed during gestation and lactation differ in effects on characteristics of the metabolic syndrome in male offspring of Wistar rats.Br J Nutr. 2012 Jan;107(2):284-94. doi: 10.1017/S0007114511002686. Epub 2011 Jun 29. Br J Nutr. 2012. PMID: 21733315
-
Postnatal prebiotic fiber intake in offspring exposed to gestational protein restriction has sex-specific effects on insulin resistance and intestinal permeability in rats.J Nutr. 2014 Oct;144(10):1556-63. doi: 10.3945/jn.114.194142. Epub 2014 Jul 30. J Nutr. 2014. PMID: 25080539
Cited by
-
Maternal High-Fat Diet Programs Offspring Liver Steatosis in a Sexually Dimorphic Manner in Association with Changes in Gut Microbial Ecology in Mice.Sci Rep. 2018 Nov 7;8(1):16502. doi: 10.1038/s41598-018-34453-0. Sci Rep. 2018. PMID: 30405201 Free PMC article.
-
Paternal phthalate exposure-elicited offspring metabolic disorders are associated with altered sperm small RNAs in mice.Environ Int. 2023 Feb;172:107769. doi: 10.1016/j.envint.2023.107769. Epub 2023 Jan 23. Environ Int. 2023. PMID: 36709676 Free PMC article.
-
Personalized Nutrition in the Management of Female Infertility: New Insights on Chronic Low-Grade Inflammation.Nutrients. 2022 May 3;14(9):1918. doi: 10.3390/nu14091918. Nutrients. 2022. PMID: 35565885 Free PMC article. Review.
-
Effects of methyl donor diets on incisional pain in mice.PLoS One. 2013 Oct 24;8(10):e77881. doi: 10.1371/journal.pone.0077881. eCollection 2013. PLoS One. 2013. PMID: 24205011 Free PMC article.
-
Pregnancy homocysteine and cobalamin status predict childhood metabolic health in the offspring.Pediatr Res. 2023 Feb;93(3):633-642. doi: 10.1038/s41390-022-02117-5. Epub 2022 May 31. Pediatr Res. 2023. PMID: 35641553
References
-
- Hales CN, Ozanne SE. For debate: fetal and early postnatal growth restriction lead to diabetes, the metabolic syndrome and renal failure. Diabetologia. 2003;46:1013–9 - PubMed
-
- Fleming TP, Kwong WY, Porter R, Ursell E, Fesenko I, Wilkins A, Miller DJ, Watkins AJ, Eckert JJ. The embryo and its future. Biol Reprod. 2004;71:1046–54 - PubMed
-
- Duranthon V, Watson AJ, Lonergan P. Preimplantation embryo programming: transcription, epigenetics, and culture environment. Reproduction. 2008;135:141–50 - PubMed
-
- de Rooij SR, Painter RC, Phillips DIW, Osmond C, Michels RPJ, Godsland IF, Bossuyt PMM, Bleker OP, Roseboom TJ. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006;29:1897–901 - PubMed
-
- Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, Thurston A, Huntley JF, Rees WD, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. 2007;104:19351–6 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

