A dynamic notch injury response activates epicardium and contributes to fibrosis repair
- PMID: 21106942
- PMCID: PMC3042596
- DOI: 10.1161/CIRCRESAHA.110.233262
A dynamic notch injury response activates epicardium and contributes to fibrosis repair
Abstract
Rationale: Transgenic Notch reporter mice express enhanced green fluorescent protein in cells with C-promoter binding factor-1 response element transcriptional activity (CBF1-RE(x)₄-EGFP), providing a unique and powerful tool for identifying and isolating "Notch-activated" progenitors.
Objective: We asked whether, as in other tissues of this mouse, EGFP localized and functionally tagged adult cardiac tissue progenitors, and, if so, whether this cell-based signal could serve as a quantitative and qualitative biosensor of the injury repair response of the heart.
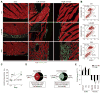
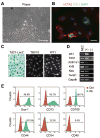
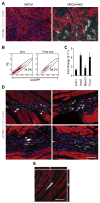
Methods and results: In addition to scattered endothelial and interstitial cells, Notch-activated (EGFP(+)) cells unexpectedly richly populated the adult epicardium. We used fluorescence-activated cell sorting to isolate EGFP(+) cells and excluded hematopoietic (CD45(+)) and endothelial (CD31(+)) subsets. We analyzed EGFP(+)/CD45⁻/CD31⁻ cells, a small (<2%) but distinct subpopulation, by gene expression profiling and functional analyses. We called this mixed cell pool, which had dual multipotent stromal cell and epicardial lineage signatures, Notch-activated epicardial-derived cells (NECs). Myocardial infarction and thoracic aortic banding amplified the NEC pool, increasing fibroblast differentiation. Validating the functional vitality of clonal NEC lines, serum growth factors triggered epithelial-mesenchymal transition and the immobilized Notch ligand Delta-like 1-activated downstream target genes. Moreover, cardiomyocyte coculture and engraftment in NOD-SCID (nonobese diabetic-severe combined immunodeficiency) mouse myocardium increased cardiac gene expression in NECs.
Conclusions: A dynamic Notch injury response activates adult epicardium, producing a multipotent cell population that contributes to fibrosis repair.
Figures



Comment in
-
Kicking the epicardium up a notch.Circ Res. 2011 Jan 7;108(1):6-8. doi: 10.1161/CIRCRESAHA.110.237297. Circ Res. 2011. PMID: 21212389 Free PMC article. No abstract available.
References
-
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

