Oral resveratrol reduces neuronal damage in a model of multiple sclerosis
- PMID: 21107122
- PMCID: PMC3312784
- DOI: 10.1097/WNO.0b013e3181f7f833
Oral resveratrol reduces neuronal damage in a model of multiple sclerosis
Abstract
Background: Neuronal loss in multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE), correlates with permanent neurological dysfunction. Current MS therapies have limited the ability to prevent neuronal damage.
Methods: We examined whether oral therapy with SRT501, a pharmaceutical grade formulation of resveratrol, reduces neuronal loss during relapsing-remitting EAE. Resveratrol activates SIRT1, an NAD+-dependent deacetylase that promotes mitochondrial function.
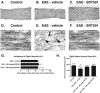
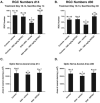
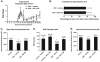
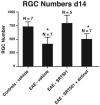
Results: Oral SRT501 prevented neuronal loss during optic neuritis, an inflammatory optic nerve lesion in MS and EAE. SRT501 also suppressed neurological dysfunction during EAE remission, and spinal cords from SRT501-treated mice had significantly higher axonal density than vehicle-treated mice. Similar neuroprotection was mediated by SRT1720, another SIRT1-activating compound; and sirtinol, an SIRT1 inhibitor, attenuated SRT501 neuroprotective effects. SIRT1 activators did not prevent inflammation.
Conclusions: These studies demonstrate that SRT501 attenuates neuronal damage and neurological dysfunction in EAE by a mechanism involving SIRT1 activation. SIRT1 activators are a potential oral therapy in MS.
Figures




Comment in
-
Preserving and restoring optic nerve function.J Neuroophthalmol. 2010 Dec;30(4):303-4. doi: 10.1097/WNO.0b013e318200de91. J Neuroophthalmol. 2010. PMID: 21107120 No abstract available.
References
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Eng J Med. 2002;343:938–952. - PubMed
-
- Davie CA, Barker GJ, Webb S, Tofts PS, Thompson AJ, Harding AE, McDonald WI, Miller DH. Persistent functional deficit in multiple sclerosis and autosomal dominant ataxia associated with axon loss. Brain. 1995;118:1583–1592. - PubMed
-
- Losseff NA, Webb SL, O'Riordan JI, Page R, Wang L, Barker GJ, Tofts PS, McDonald WI, Miller DH, Thompson AJ. Spinal cord atrophy and disability in multiple sclerosis: a new reproducible and sensitive MRI method with potential to monitor disease progression. Brain. 1996;119:701–708. - PubMed
-
- Losseff NA, Wang L, Lai HM, Yoo DS, Gawne-Cain ML, McDonald WI, Miller DH, Thompson AJ. Progressive cerebral atrophy in multiple sclerosis: a serial MRI study. Brain. 1996;119:2009–2019. - PubMed
-
- Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmol. 2006;113:324–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

