An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla
- PMID: 21107206
- PMCID: PMC3124849
- DOI: 10.1227/NEU.0b013e3181f74105
An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla
Abstract
Background: Deep brain stimulation (DBS) surgery is used for treating movement disorders, including Parkinson disease, essential tremor, and dystonia. Successful DBS surgery is critically dependent on precise placement of DBS electrodes into target structures. Frequently, DBS surgery relies on normalized atlas-derived diagrams that are superimposed on patient brain magnetic resonance imaging (MRI) scans, followed by microelectrode recording and macrostimulation to refine the ultimate electrode position. Microelectrode recording carries a risk of hemorrhage and requires active patient participation during surgery.
Objective: To enhance anatomic imaging for DBS surgery using high-field MRI with the ultimate goal of improving the accuracy of anatomic target selection.
Methods: Using a 7-T MRI scanner combined with an array of acquisition schemes using multiple image contrasts, we obtained high-resolution images of human deep nuclei in healthy subjects.
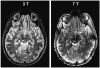
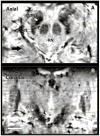
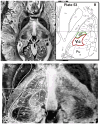
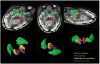
Results: Superior image resolution and contrast obtained at 7 T in vivo using susceptibility-weighted imaging dramatically improved anatomic delineation of DBS targets and allowed the identification of internal architecture within these targets. A patient-specific, 3-dimensional model of each target area was generated on the basis of the acquired images.
Conclusion: Technical developments in MRI at 7 T have yielded improved anatomic resolution of deep brain structures, thereby holding the promise of improving anatomic-based targeting for DBS surgery. Future study is needed to validate this technique in improving the accuracy of targeting in DBS surgery.
Figures



References
-
- Starr PA. Placement of deep brain stimulators into the subthalamic nucleus or globus pallidus internus: technical approach. Stereotact Funct Neurosurg. 2002;79(3-4):118–145. - PubMed
-
- Rijkers K, Temel Y, Visser-Vandewalle V, et al. The microanatomical environment of the subthalamic nucleus: technical note. J Neurosurg. 2007;107(1):198–201. - PubMed
-
- Patel NK, Khan S, Gill SS. Comparison of atlas- and magnetic-resonance-imaging-based stereotactic targeting of the subthalamic nucleus in the surgical treatment of Parkinson's disease. Stereotact Funct Neurosurg. 2008;86(3):153–161. - PubMed
-
- Ashkan K, Blomstedt P, Zrinzo L, et al. Variability of the subthalamic nucleus: the case for direct MRI guided targeting. Br J Neurosurg. 2007;21(2):197–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

