Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration
- PMID: 21107266
- PMCID: PMC3104241
- DOI: 10.1097/QAI.0b013e3182060610
Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration
Abstract
Background: With expanding pediatric antiretroviral therapy (ART) access, children will begin to experience treatment failure and require second-line therapy. We evaluated the probability and determinants of virologic failure and switching in children in South Africa.
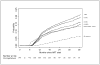
Methods: Pooled analysis of routine individual data from children who initiated ART in 7 South African treatment programs with 6-monthly viral load and CD4 monitoring produced Kaplan-Meier estimates of probability of virologic failure (2 consecutive unsuppressed viral loads with the second being >1000 copies/mL, after ≥24 weeks of therapy) and switch to second-line. Cox-proportional hazards models stratified by program were used to determine predictors of these outcomes.
Results: The 3-year probability of virologic failure among 5485 children was 19.3% (95% confidence interval: 17.6 to 21.1). Use of nevirapine or ritonavir alone in the initial regimen (compared with efavirenz) and exposure to prevention of mother to child transmission regimens were independently associated with failure [adjusted hazard ratios (95% confidence interval): 1.77 (1.11 to 2.83), 2.39 (1.57 to 3.64) and 1.40 (1.02 to 1.92), respectively]. Among 252 children with ≥1 year follow-up after failure, 38% were switched to second-line. Median (interquartile range) months between failure and switch was 5.7 (2.9-11.0).
Conclusions: Triple ART based on nevirapine or ritonavir as a single protease inhibitor seems to be associated with a higher risk of virologic failure. A low proportion of virologically failing children were switched.
Figures
References
-
- The KIDS-ART-LINC Collaboration. Low risk of Death, but Substantial Program Attrition, in Pediatric Treatment Cohorts in Sub-Saharan Africa. J Aquir Immune Defic Syndr. 2008;15(5):523–531. - PubMed
-
- WHO. Antiretroviral therapy of HIV infection in infants and children: towards universal access. 2006 . [Accessed 2007/09/02.]. http://www.who.int/hiv/pub/paediatric/infants/en/index.html. - PubMed
-
- Paediatric European Network for Treatment of Aids. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. 2008. [Accessed 2009/11/01]. http://www.pentatrials.org/guide09.pdf.
-
- Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in Pediatric HIV infection. 2008 . [Accessed 2008/12/17.]. http://AIDSinfo.nih.gov. - PubMed
-
- Jittamala P, Puthanakit T, Chaiinseeard S, et al. Predictors of virologic failure and genotypic resistance mutation patterns in Thai children receiving non0nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J. 2009;28(9):826–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

