Drug metabolism within the brain changes drug response: selective manipulation of brain CYP2B alters propofol effects
- PMID: 21107310
- PMCID: PMC3055692
- DOI: 10.1038/npp.2010.202
Drug metabolism within the brain changes drug response: selective manipulation of brain CYP2B alters propofol effects
Abstract
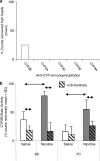
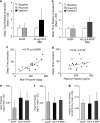
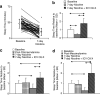
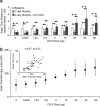
Drug-metabolizing cytochrome P450 (CYPs) enzymes are expressed in the liver, as well as in extrahepatic tissues such as the brain. Here we show for the first time that drug metabolism by a CYP within the brain, illustrated using CYP2B and the anesthetic propofol (2, 6-diisopropylphenol, Diprivan), can meaningfully alter the pharmacological response to a CNS acting drug. CYP2B is expressed in the brains of animals and humans, and this CYP isoform is able to metabolize centrally acting substrates such as propofol, ecstasy, and serotonin. Rats were given intracerebroventricularly (i.c.v.) injections of vehicle, C8-xanthate, or 8-methoxypsoralen (CYP2B mechanism-based inhibitors) and then tested for sleep time following propofol (80 mg/kg intraperitoneally). Both inhibitors significantly increased sleep-time (1.8- to 2-fold) and brain propofol levels, while having no effect on plasma propofol levels. Seven days of nicotine treatment can induce the expression of brain, but not hepatic, CYP2B, and this induction reduced propofol sleep times by 2.5-fold. This reduction was reversed in a dose-dependent manner by i.c.v. injections of inhibitor. Sleep times correlated with brain (r=0.76, P=0.0009), but not plasma (r=0.24, P=0.39) propofol concentrations. Inhibitor treatments increased brain, but not plasma, propofol levels, and had no effect on hepatic enzyme activity. These data indicate that brain CYP2B can metabolize neuroactive substrates (eg, propofol) and can alter their pharmacological response. This has wider implications for localized CYP-mediated metabolism of drugs, neurotransmitters, and neurotoxins within the brain by this highly variable enzyme family and other CYP subfamilies expressed in the brain.
Figures

Similar articles
-
Rat brain CYP2B induction by nicotine is persistent and does not involve nicotinic acetylcholine receptors.Brain Res. 2010 Aug 12;1348:1-9. doi: 10.1016/j.brainres.2010.06.035. Epub 2010 Jun 20. Brain Res. 2010. PMID: 20599831
-
Brain CYP2B induction can decrease nicotine levels in the brain.Addict Biol. 2017 Sep;22(5):1257-1266. doi: 10.1111/adb.12411. Epub 2016 May 27. Addict Biol. 2017. PMID: 27230546 Free PMC article.
-
Effect of Brain CYP2B Inhibition on Brain Nicotine Levels and Nicotine Self-Administration.Neuropsychopharmacology. 2015 Jul;40(8):1910-8. doi: 10.1038/npp.2015.40. Epub 2015 Feb 5. Neuropsychopharmacology. 2015. PMID: 25652250 Free PMC article.
-
Regional and cellular induction of nicotine-metabolizing CYP2B1 in rat brain by chronic nicotine treatment.Biochem Pharmacol. 2000 Jun 15;59(12):1501-11. doi: 10.1016/s0006-2952(00)00281-1. Biochem Pharmacol. 2000. PMID: 10799646
-
Cytochrome P450-mediated drug metabolism in the brain.J Psychiatry Neurosci. 2013 May;38(3):152-63. doi: 10.1503/jpn.120133. J Psychiatry Neurosci. 2013. PMID: 23199531 Free PMC article. Review.
Cited by
-
First demonstration that brain CYP2D-mediated opiate metabolic activation alters analgesia in vivo.Biochem Pharmacol. 2013 Jun 15;85(12):1848-55. doi: 10.1016/j.bcp.2013.04.014. Epub 2013 Apr 23. Biochem Pharmacol. 2013. PMID: 23623752 Free PMC article.
-
Nicotine regulates the expression of UDP-glucuronosyltransferase (UGT) in humanized UGT1 mouse brain.Drug Metab Pharmacokinet. 2015 Aug;30(4):269-75. doi: 10.1016/j.dmpk.2015.04.004. Epub 2015 May 7. Drug Metab Pharmacokinet. 2015. PMID: 26210671 Free PMC article.
-
"Target-Site" Drug Metabolism and Transport.Drug Metab Dispos. 2015 Aug;43(8):1156-68. doi: 10.1124/dmd.115.064576. Epub 2015 May 18. Drug Metab Dispos. 2015. PMID: 25986849 Free PMC article.
-
Impact of nicotine metabolism on nicotine's pharmacological effects and behavioral responses: insights from a Cyp2a(4/5)bgs-null mouse.J Pharmacol Exp Ther. 2013 Dec;347(3):746-54. doi: 10.1124/jpet.113.208256. Epub 2013 Sep 17. J Pharmacol Exp Ther. 2013. PMID: 24045421 Free PMC article.
-
Drug and xenobiotic biotransformation in the blood-brain barrier: a neglected issue.Front Cell Neurosci. 2014 Oct 17;8:335. doi: 10.3389/fncel.2014.00335. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25368552 Free PMC article.
References
-
- Albores A, Ortega-Mantilla G, Sierra-Santoyo A, Cebrian ME, Munoz-Sanchez JL, Calderon-Salinas JV, et al. Cytochrome P450 2B (CYP2B)-mediated activation of methyl-parathion in rat brain extracts. Toxicol Lett. 2001;124:1–10. - PubMed
-
- Altomare C, Trapani G, Latrofa A, Serra M, Sanna E, Biggio G, et al. Highly water-soluble derivatives of the anesthetic agent propofol: in vitro and in vivo evaluation of cyclic amino acid esters. Eur J Pharm Sci. 2003;20:17–26. - PubMed
-
- Bhargava VK, Saha L. Cholinergic mechanism in imipramine and morphine antinoception. Boll Chim Farm. 2001;140:201–204. - PubMed
-
- Bijl MJ, Luijendijk HJ, van den Berg JF, Visser LE, van Schaik RH, Hofman A, et al. Association between the CYP2D6*4 polymorphism and depression or anxiety in the elderly. Pharmacogenomics. 2009;10:541–547. - PubMed
-
- Bromek E, Haduch A, Daniel WA. The ability of cytochrome P450 2D isoforms to synthesize dopamine in the brain: an in vitro study. Eur J Pharmacol. 2010;626:171–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

