Nanoscale architecture of integrin-based cell adhesions
- PMID: 21107430
- PMCID: PMC3046339
- DOI: 10.1038/nature09621
Nanoscale architecture of integrin-based cell adhesions
Abstract
Cell adhesions to the extracellular matrix (ECM) are necessary for morphogenesis, immunity and wound healing. Focal adhesions are multifunctional organelles that mediate cell-ECM adhesion, force transmission, cytoskeletal regulation and signalling. Focal adhesions consist of a complex network of trans-plasma-membrane integrins and cytoplasmic proteins that form a <200-nm plaque linking the ECM to the actin cytoskeleton. The complexity of focal adhesion composition and dynamics implicate an intricate molecular machine. However, focal adhesion molecular architecture remains unknown. Here we used three-dimensional super-resolution fluorescence microscopy (interferometric photoactivated localization microscopy) to map nanoscale protein organization in focal adhesions. Our results reveal that integrins and actin are vertically separated by a ∼40-nm focal adhesion core region consisting of multiple protein-specific strata: a membrane-apposed integrin signalling layer containing integrin cytoplasmic tails, focal adhesion kinase and paxillin; an intermediate force-transduction layer containing talin and vinculin; and an uppermost actin-regulatory layer containing zyxin, vasodilator-stimulated phosphoprotein and α-actinin. By localizing amino- and carboxy-terminally tagged talins, we reveal talin's polarized orientation, indicative of a role in organizing the focal adhesion strata. The composite multilaminar protein architecture provides a molecular blueprint for understanding focal adhesion functions.
Figures
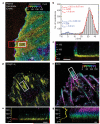
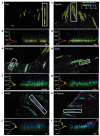
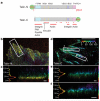
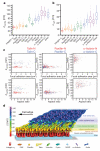
Comment in
-
Cell adhesion: Getting to the core.Nat Rev Mol Cell Biol. 2011 Jan;12(1):4. doi: 10.1038/nrm3030. Epub 2010 Dec 1. Nat Rev Mol Cell Biol. 2011. PMID: 21119700 No abstract available.
References
-
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–518. - PubMed
-
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton. Nature Rev. Mol. Cell Biol. 2001;2:793–805. - PubMed
-
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003;19:677–695. - PubMed
-
- Franz CM, Muller DJ. Analyzing focal adhesion structure by atomic force microscopy. J. Cell Sci. 2005;118:5315–5323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

