Alkynyl-farnesol reporters for detection of protein S-prenylation in cells
- PMID: 21107478
- PMCID: PMC4231476
- DOI: 10.1039/c0mb00183j
Alkynyl-farnesol reporters for detection of protein S-prenylation in cells
Abstract
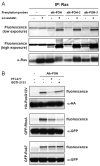
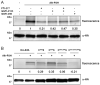
Protein S-prenylation is a lipid modification that regulates membrane-protein and protein-protein interactions in cell signaling. Though sites of protein S-prenylation can be predicted based upon conserved C-terminal CaaX or CC/CXC motifs, biochemical detection of protein S-prenylation in cells is still challenging. Herein, we report an alkynyl-isoprenol chemical reporter (alk-FOH) as an efficient substrate for prenyltransferases in mammalian cells that enables sensitive detection of S-farnesylated and S-geranylgeranylated proteins using bioorthogonal ligation methods. Fluorescent detection alleviates the need to deplete cellular isoprenoids for biochemical analysis of S-prenylated proteins and enables robust characterization of S-prenylated proteins, such as effectors that are injected into host cells by bacterial pathogens. This alkynyl-prenylation reporter provides a sensitive tool for biochemical analysis and rapid profiling of prenylated proteins in cells.
Figures


Similar articles
-
Consequences of altered isoprenylation targets on a-factor export and bioactivity.Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1275-9. doi: 10.1073/pnas.91.4.1275. Proc Natl Acad Sci U S A. 1994. PMID: 8108401 Free PMC article.
-
Prenylome profiling reveals S-farnesylation is crucial for membrane targeting and antiviral activity of ZAP long-isoform.Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11085-90. doi: 10.1073/pnas.1302564110. Epub 2013 Jun 17. Proc Natl Acad Sci U S A. 2013. PMID: 23776219 Free PMC article.
-
Farnesol-mediated shift in the metabolic origin of prenyl groups used for protein prenylation in plants.Biochimie. 2016 Aug;127:95-102. doi: 10.1016/j.biochi.2016.04.021. Epub 2016 Apr 29. Biochimie. 2016. PMID: 27138105
-
Isoprenoids and protein prenylation: implications in the pathogenesis and therapeutic intervention of Alzheimer's disease.Crit Rev Biochem Mol Biol. 2018 Jun;53(3):279-310. doi: 10.1080/10409238.2018.1458070. Crit Rev Biochem Mol Biol. 2018. PMID: 29718780 Free PMC article. Review.
-
Recent advances in protein prenyltransferases: substrate identification, regulation, and disease interventions.Curr Opin Chem Biol. 2012 Dec;16(5-6):544-52. doi: 10.1016/j.cbpa.2012.10.015. Epub 2012 Nov 8. Curr Opin Chem Biol. 2012. PMID: 23141597 Free PMC article. Review.
Cited by
-
Chemical reporters for biological discovery.Nat Chem Biol. 2013 Aug;9(8):475-84. doi: 10.1038/nchembio.1296. Nat Chem Biol. 2013. PMID: 23868317 Free PMC article. Review.
-
A novel role of farnesylation in targeting a mitotic checkpoint protein, human Spindly, to kinetochores.J Cell Biol. 2015 Mar 30;208(7):881-96. doi: 10.1083/jcb.201412085. J Cell Biol. 2015. PMID: 25825516 Free PMC article.
-
An Alkyne-Containing Isoprenoid Analogue Based on a Farnesyl Diphosphate Scaffold Is a Biologically Functional Universal Probe for Proteomic Analysis.Biochemistry. 2025 Jan 7;64(1):138-155. doi: 10.1021/acs.biochem.4c00558. Epub 2024 Dec 9. Biochemistry. 2025. PMID: 39652878
-
S-palmitoylation and ubiquitination differentially regulate interferon-induced transmembrane protein 3 (IFITM3)-mediated resistance to influenza virus.J Biol Chem. 2012 Jun 1;287(23):19631-41. doi: 10.1074/jbc.M112.362095. Epub 2012 Apr 17. J Biol Chem. 2012. PMID: 22511783 Free PMC article.
-
A Not-So-Ancient Grease History: Click Chemistry and Protein Lipid Modifications.Chem Rev. 2021 Jun 23;121(12):7178-7248. doi: 10.1021/acs.chemrev.0c01108. Epub 2021 Apr 6. Chem Rev. 2021. PMID: 33821625 Free PMC article. Review.
References
-
- Resh MD. Nat Chem Biol. 2006;2:584–590. - PubMed
-
- Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Nat Rev Drug Discov. 2007;6:541–555. - PubMed
-
- Eastman RT, Buckner FS, Yokoyama K, Gelb MH, Van Voorhis WC. J Lipid Res. 2006;47:233–240. - PubMed
-
- Lobell RB. Adv Immunol. 1998;68:145–189. - PubMed
-
- Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. Science. 2005;307:1746–1752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

