Dissociation of lipotoxicity and glucotoxicity in a mouse model of obesity associated diabetes: role of forkhead box O1 (FOXO1) in glucose-induced beta cell failure
- PMID: 21107520
- PMCID: PMC3034032
- DOI: 10.1007/s00125-010-1973-8
Dissociation of lipotoxicity and glucotoxicity in a mouse model of obesity associated diabetes: role of forkhead box O1 (FOXO1) in glucose-induced beta cell failure
Abstract
Aims/hypothesis: Carbohydrate-free diet prevents hyperglycaemia and beta cell destruction in the New Zealand Obese (NZO) mouse model. Here we have used a sequential dietary regimen to dissociate the effects of obesity and hyperglycaemia on beta cell function and integrity, and to study glucose-induced alterations of key transcription factors over 16 days.
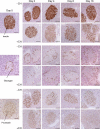
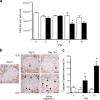
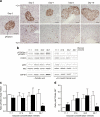
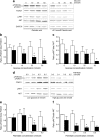
Methods: Mice were rendered obese by feeding a carbohydrate-free diet for 18 weeks. Thereafter, a carbohydrate-containing diet was given. Plasma glucose, plasma insulin and total pancreatic insulin were determined, and forkhead box O1 protein (FOXO1) phosphorylation and the transcription factors pancreatic and duodenal homeobox 1 (PDX1), NK6 homeobox 1 protein (NKX6.1) and v-maf musculoaponeurotic fibrosarcoma oncogene family, protein A (avian) (MAFA) were monitored by immunohistochemistry for 16 days.
Results: Dietary carbohydrates produced a rapid and continuous increase in plasma glucose in NZO mice between day 2 and 16 after the dietary challenge. Hyperglycaemia caused a dramatic dephosphorylation of FOXO1 at day 2, followed by a progressive depletion of insulin stores. The loss of beta cells was triggered by apoptosis (detectable at day 8), associated with reduction of crucial transcription factors (PDX1, NKX6.1 and MAFA). Incubation of isolated islets from carbohydrate-restricted NZO mice or MIN6 cells with palmitate and glucose for 48 h resulted in a dephosphorylation of FOXO1 and thymoma viral proto-oncogene 1 (AKT) without changing the protein levels of both proteins.
Conclusions/interpretation: The dietary regimen dissociates the effects of obesity (lipotoxicity) from those of hyperglycaemia (glucotoxicity) in NZO mice. Obese NZO mice are unable to compensate for the carbohydrate challenge by increasing insulin secretion or synthesising adequate amounts of insulin. In response to the hyperglycaemia, FOXO1 is dephosphorylated, leading to reduced levels of beta cell-specific transcription factors and to apoptosis of the cells.
Figures




References
-
- Herberg L, Leiter EH. Obesity/diabetes in mice with mutations in the leptin or leptin receptor genes. In: Sima AAF, Shafrir E, editors. Frontiers in animal diabetes research, vol. 2: animal models of diabetes. Amsterdam: Harwood Academic Publishers; 2001. pp. 63–107.
-
- Plum L, Kluge R, Giesen K, Altmüller J, Ortlepp JR, Joost HG. Type-2-diabetes-like hyperglycaemia in a backcross model of New Zealand obese (NZO) and SJL mice: characterization of a susceptibility locus on chromosome 4 and its relation with obesity. Diabetes. 2000;49:1590–1596. doi: 10.2337/diabetes.49.9.1590. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

