Structural insights into the function of P2X4: an ATP-gated cation channel of neuroendocrine cells
- PMID: 21107680
- PMCID: PMC3042234
- DOI: 10.1007/s10571-010-9568-y
Structural insights into the function of P2X4: an ATP-gated cation channel of neuroendocrine cells
Abstract
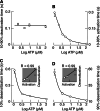
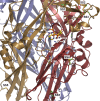
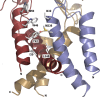
The P2X4 receptor (P2X4R) is a member of a family of ATP-gated cation channels that are composed of three subunits. Each subunit has two transmembrane (TM) domains linked by a large extracellular loop and intracellularly located N- and C-termini. The receptors are expressed in excitable and non-excitable cells and have been implicated in the modulation of membrane excitability, calcium signaling, neurotransmitter and hormone release, and pain physiology. P2X4Rs activate rapidly and desensitize within the seconds of agonist application, both with the rates dependent on ATP concentrations, and deactivate rapidly and independently of ATP concentration. Disruption of conserved cysteine ectodomain residues affects ATP binding and gating. Several ectodomain residues of P2X4R were identified as critical for ATP binding, including K67, K313, and R295. Ectodomain residues also account for the allosteric regulation of P2X4R; H140 is responsible for copper binding and H286 regulates receptor functions with protons. Ivermectin sensitized receptors, amplified the current amplitude, and slowed receptor deactivation by binding in the TM region. Scanning mutagenesis of TMs revealed the helical topology of both domains, and suggested that receptor function is critically dependent on the conserved Y42 residue. In this brief article, we summarize this study and re-interpret it using a model based on crystallization of the zebrafish P2X4.1 receptor.
Figures


Similar articles
-
Multiple roles of the extracellular vestibule amino acid residues in the function of the rat P2X4 receptor.PLoS One. 2013;8(3):e59411. doi: 10.1371/journal.pone.0059411. Epub 2013 Mar 21. PLoS One. 2013. PMID: 23555667 Free PMC article.
-
Identification of functionally important residues of the rat P2X4 receptor by alanine scanning mutagenesis of the dorsal fin and left flipper domains.PLoS One. 2014 Nov 14;9(11):e112902. doi: 10.1371/journal.pone.0112902. eCollection 2014. PLoS One. 2014. PMID: 25398027 Free PMC article.
-
Deciphering the regulation of P2X4 receptor channel gating by ivermectin using Markov models.PLoS Comput Biol. 2017 Jul 14;13(7):e1005643. doi: 10.1371/journal.pcbi.1005643. eCollection 2017 Jul. PLoS Comput Biol. 2017. PMID: 28708827 Free PMC article.
-
Structural interpretation of P2X receptor mutagenesis studies on drug action.Br J Pharmacol. 2010 Nov;161(5):961-71. doi: 10.1111/j.1476-5381.2010.00728.x. Br J Pharmacol. 2010. PMID: 20977449 Free PMC article. Review.
-
The role of P2X4 receptors in chronic pain: A potential pharmacological target.Biomed Pharmacother. 2020 Sep;129:110447. doi: 10.1016/j.biopha.2020.110447. Epub 2020 Jul 3. Biomed Pharmacother. 2020. PMID: 32887026 Review.
Cited by
-
Multiple roles of the extracellular vestibule amino acid residues in the function of the rat P2X4 receptor.PLoS One. 2013;8(3):e59411. doi: 10.1371/journal.pone.0059411. Epub 2013 Mar 21. PLoS One. 2013. PMID: 23555667 Free PMC article.
-
ATP binding site mutagenesis reveals different subunit stoichiometry of functional P2X2/3 and P2X2/6 receptors.J Biol Chem. 2012 Apr 20;287(17):13930-43. doi: 10.1074/jbc.M112.345207. Epub 2012 Feb 29. J Biol Chem. 2012. PMID: 22378790 Free PMC article.
-
Elevated hydrostatic pressure stimulates ATP release which mediates activation of the NLRP3 inflammasome via P2X4 in rat urothelial cells.Int Urol Nephrol. 2018 Sep;50(9):1607-1617. doi: 10.1007/s11255-018-1948-0. Epub 2018 Aug 11. Int Urol Nephrol. 2018. PMID: 30099658 Free PMC article.
-
Regulation of ATP-gated P2X channels: from redox signaling to interactions with other proteins.Antioxid Redox Signal. 2014 Aug 20;21(6):953-70. doi: 10.1089/ars.2013.5549. Epub 2013 Sep 25. Antioxid Redox Signal. 2014. PMID: 23944253 Free PMC article. Review.
-
Allosteric regulation of the P2X4 receptor channel pore dilation.Pflugers Arch. 2015 Apr;467(4):713-26. doi: 10.1007/s00424-014-1546-7. Epub 2014 Jun 11. Pflugers Arch. 2015. PMID: 24917516 Free PMC article.
References
-
- Acuna-Castillo C, Morales B, Huidobro-Toro JP (2000) Zinc and copper modulate differentially the P2X4 receptor. J Neurochem 74:1529–1537 - PubMed
-
- Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R (1995) A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett 375:129–133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

