Robust genital gag-specific CD8+ T-cell responses in mice upon intramuscular immunization with simian adenoviral vectors expressing HIV-1-gag
- PMID: 21108465
- PMCID: PMC3154578
- DOI: 10.1002/eji.201040440
Robust genital gag-specific CD8+ T-cell responses in mice upon intramuscular immunization with simian adenoviral vectors expressing HIV-1-gag
Abstract
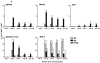
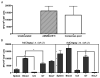
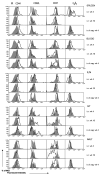
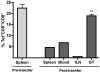
Most studies on E1-deleted adenovirus (Ad) vectors as vaccine carriers for antigens of HIV-1 have focused on induction of central immune responses, although stimulation of mucosal immunity at the genital tract (GT), the primary port of entry of HIV-1, would also be highly desirable. In this study, different immunization protocols using chimpanzee-derived adenoviral (AdC) vectors expressing Gag of HIV-1 clade B given in heterologous prime-boost regimens were tested for induction of systemic and genital immune responses. Although i.n. immunization stimulated CD8(+) T-cell responses that could be detected in the GT, this route induced only marginal cellular responses in systemic tissues and furthermore numbers of Gag-specific CD8(+) T cells contracted sharply within a few weeks. On the contrary, i.m. immunization induced higher and more sustained frequencies of vaccine-induced cells which could be detected in the GT as well as systemic compartments. Antigen-specific CD8(+) T cells could be detected 1 year after immunization in all compartments analyzed. Genital memory cells secreted IFN-γ, expressed high levels of CD103 and their phenotypes were consistent with a state of activation. Taken together, the results presented here show that i.m. vaccination with chimpanzee-derived (simian) adenovirus vectors is a suitable strategy to induce a long-lived genital CD8(+) T-cell response.
Copyright © 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Figures




References
-
- Pikora CA. Glycosylation of the ENV spike of primate immunodeficiency viruses and antibody neutralization. Curr HIV Res. 2004;2:243–254. - PubMed
-
- Jones PL, Korte T, Blumenthal R. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J Biol Chem. 1998;273:404–409. - PubMed
-
- McMichael AJ. HIV vaccines. Annu Rev Immunol. 2006;24:227–255. - PubMed
-
- Chan DJ. Fatal attraction: sex, sexually transmitted infections and HIV-1. Int J STD AIDS. 2006;17:643–651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

