Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor
- PMID: 21108726
- PMCID: PMC3042147
- DOI: 10.1111/j.1474-9726.2010.00653.x
Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor
Abstract
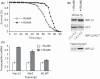
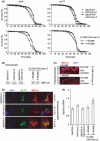
Mammals possess multiple insulin-like growth factor (IGF) binding proteins (IGFBPs), and related proteins, that modulate the activity of insulin/IGF signalling (IIS), a conserved neuroendocrine signalling pathway that affects animal lifespan. Here, we examine if increased levels of an IGFBP-like protein can extend lifespan, using Drosophila as the model organism. We demonstrate that Imaginal morphogenesis protein-Late 2 (IMP-L2), a secreted protein and the fly homologue of the human IGFBP7 tumour suppressor, is capable of binding at least two of the seven Drosophila insulin-like peptides (DILPs), namely native DILP2 and DILP5 as present in the adult fly. Increased expression of Imp-L2 results in phenotypic changes in the adult consistent with down-regulation of IIS, including accumulation of eIF-4E binding protein mRNA, increase in storage lipids, reduced fecundity and enhanced oxidative stress resistance. Increased Imp-L2 results in up-regulation of dilp2, dilp3 and dilp5 mRNA, revealing a feedback circuit that is mediated via the fly gut and/or fat body. Importantly, over-expression of Imp-L2, ubiquitous or restricted to DILP-producing cells or gut and fat body, extends lifespan. This enhanced longevity can also be observed upon adult-onset induction of Imp-L2, indicating it is not attributable to developmental changes. Our findings point to the possibility that an IGFBP or a related protein, such as IGFBP7, plays a role in mammalian aging.
Figures





References
-
- Arquier N, Geminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Leopold P. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab. 2008;7:333–338. - PubMed
-
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. - PubMed
-
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl Acad. Sci. USA. 2005;102:3105–3110. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

