Age-related alterations in the dynamic behavior of microglia
- PMID: 21108733
- PMCID: PMC3056927
- DOI: 10.1111/j.1474-9726.2010.00660.x
Age-related alterations in the dynamic behavior of microglia
Abstract
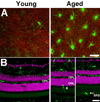
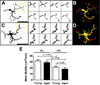
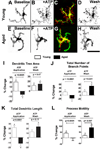
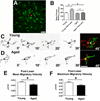
Microglia, the primary resident immune cells of the central nervous system (CNS), exhibit dynamic behavior involving rapid process motility and cellular migration that is thought to underlie key functions of immune surveillance and tissue repair. Although age-related changes in microglial activation have been implicated in the pathogenesis of neurodegenerative diseases of aging, how dynamic behavior in microglia is influenced by aging is not fully understood. In this study, we employed live imaging of retinal microglia in situ to compare microglial morphology and behavioral dynamics in young and aged animals. We found that aged microglia in the resting state have significantly smaller and less branched dendritic arbors, and also slower process motilities, which probably compromise their ability to survey and interact with their environment continuously. We also found that dynamic microglial responses to injury were age-dependent. While young microglia responded to extracellular ATP, an injury-associated signal, by increasing their motility and becoming more ramified, aged microglia exhibited a contrary response, becoming less dynamic and ramified. In response to laser-induced focal tissue injury, aged microglia demonstrated slower acute responses with lower rates of process motility and cellular migration compared with young microglia. Interestingly, the longer term response of disaggregation from the injury site was retarded in aged microglia, indicating that senescent microglial responses, while slower to initiate, are more sustained. Together, these altered features of microglial behavior at rest and following injury reveal an age-dependent dysregulation of immune response in the CNS that may illuminate microglial contributions to age-related neuroinflammatory degeneration.
No claim to original US government works. Aging Cell © 2010 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures

References
-
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. - PubMed
-
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. - PubMed
-
- Birch DG, Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;110:1571–1576. - PubMed
-
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1437–1446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

