Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model
- PMID: 21108815
- PMCID: PMC3003234
- DOI: 10.1186/1743-8977-7-35
Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model
Abstract
Background: Nanotechnology and engineered nanomaterials (ENM) are here to stay. Recent evidence suggests that exposure to environmental particulate matter exacerbates symptoms of asthma. In the present study we investigated the modulatory effects of titanium dioxide particle exposure in an experimental allergic asthma.
Methods: Nonallergic (healthy) and ovalbumin-sensitized (asthmatic) mice were exposed via inhalation to two different sizes of titanium dioxide particles, nanosized (nTiO2) and fine (fTiO2), for 2 hours a day, three days a week, for four weeks at a concentration of 10 mg/m3. Different endpoints were analysed to evaluate the immunological status of the mice.
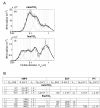
Results: Healthy mice elicited pulmonary neutrophilia accompanied by significantly increased chemokine CXCL5 expression when exposed to nTiO2. Surprisingly, allergic pulmonary inflammation was dramatically suppressed in asthmatic mice which were exposed to nTiO2 or fTiO2 particles - i.e. the levels of leucocytes, cytokines, chemokines and antibodies characteristic to allergic asthma were substantially decreased.
Conclusions: Our results suggest that repeated airway exposure to TiO2 particles modulates the airway inflammation depending on the immunological status of the exposed mice.
Figures







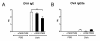
References
-
- Lötvall J, Frew A, for the European Academy of Allergology and Clinical Immunology. Allergy: an epidemic that must be stopped. Brussels: European Academy of Allergology and Clinical Immunology; 2006. http://www.eaaci.net/media/PDF/E/820.pdf
-
- Weinmayr G, Weiland SK, Bjorksten B, Brunekreef B, Buchele G, Cookson WOC, Garcia-Marcos L, Gotua M, Gratziou C, van Hage M, von Mutius E, Riikjarv M-A, Rzehak P, Stein RT, Strachan DP, Tsanakas J, Wickens K, Wong GW. Isaac Phase Two Study Group. Atopic Sensitization and the International Variation of Asthma Symptom Prevalence in Children. Am J Respir Crit Care Med. 2007;176:565–574. doi: 10.1164/rccm.200607-994OC. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

