Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers
- PMID: 21109030
- PMCID: PMC3050074
- DOI: 10.1016/j.actbio.2010.11.022
Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers
Abstract
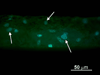
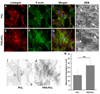
As the potential range of stem cell applications in tissue engineering continues to grow, the appropriate scaffolding choice is necessary to create tightly defined artificial microenvironments for each target organ. These microenvironments determine stem cell fate via control over differentiation. In this study we examined the specific effects of scaffold stiffness on embryonic mesenchymal progenitor cell behavior. Mechanically distinct scaffolds having identical microstructures and surface chemistries were produced utilizing core-shell electrospinning. The modulus of core-shell poly(ether sulfone)-poly(ε-caprolactone) (PES-PCL) fibers (30.6 MPa) was more than four times that of pure PCL (7.1 MPa). The results for chondrogenic and osteogenic differentiation of progenitor cells on each scaffold indicate that the lower modulus PCL fibers provided more appropriate microenvironments for chondrogenesis, evident by a marked up-regulation of chondrocytic Sox9, collagen type 2, and aggrecan gene expression and chondrocyte-specific extracellular matrix glycosaminoglycan production. In contrast, the stiffer core-shell PES-PCL fibers supported enhanced osteogenesis by promoting osteogenic Runx2, alkaline phosphatase, and osteocalcin gene expression, as well as alkaline phosphatase activity. The findings demonstrate that the microstructural stiffness/modules of a scaffold and the pliability of individual fibers may play a critical role in controlling stem cell differentiation. Regulation of cytoskeletal organization may occur via a "dynamic scaffold" leading to the subsequent intracellular signaling events that control differentiation-specific gene expression.
Copyright © 2010 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures







Similar articles
-
Nanoclay-enriched poly(ɛ-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells.Tissue Eng Part A. 2014 Aug;20(15-16):2088-101. doi: 10.1089/ten.tea.2013.0281. Epub 2014 May 19. Tissue Eng Part A. 2014. PMID: 24842693 Free PMC article.
-
Mechanically cartilage-mimicking poly(PCL-PTHF urethane)/collagen nanofibers induce chondrogenesis by blocking NF-kappa B signaling pathway.Biomaterials. 2018 Sep;178:281-292. doi: 10.1016/j.biomaterials.2018.06.023. Epub 2018 Jun 18. Biomaterials. 2018. PMID: 29945065 Free PMC article.
-
Platelet-rich fibrin-loaded PCL/chitosan core-shell fibers scaffold for enhanced osteogenic differentiation of mesenchymal stem cells.Carbohydr Polym. 2021 Oct 1;269:118351. doi: 10.1016/j.carbpol.2021.118351. Epub 2021 Jun 17. Carbohydr Polym. 2021. PMID: 34294355
-
Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering.Mater Sci Eng C Mater Biol Appl. 2020 Feb;107:110291. doi: 10.1016/j.msec.2019.110291. Epub 2019 Oct 8. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31761240 Free PMC article.
-
Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Jun;99:479-490. doi: 10.1016/j.msec.2019.01.127. Epub 2019 Jan 30. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30889723 Free PMC article.
Cited by
-
Improved cellular infiltration in electrospun fiber via engineered porosity.Tissue Eng. 2007 Sep;13(9):2249-57. doi: 10.1089/ten.2006.0306. Tissue Eng. 2007. PMID: 17536926 Free PMC article.
-
Individual cells generate their own self-reinforcing contact guidance cues through local matrix fiber remodeling.PLoS One. 2022 Mar 25;17(3):e0265403. doi: 10.1371/journal.pone.0265403. eCollection 2022. PLoS One. 2022. PMID: 35333902 Free PMC article.
-
Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration.Nanomedicine (Lond). 2013 Sep;8(9):1459-81. doi: 10.2217/nnm.13.132. Nanomedicine (Lond). 2013. PMID: 23987110 Free PMC article. Review.
-
Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid.Biomaterials. 2011 Dec;32(34):8771-82. doi: 10.1016/j.biomaterials.2011.08.073. Epub 2011 Sep 7. Biomaterials. 2011. PMID: 21903262 Free PMC article. Review.
-
Development and Utilization of Multifunctional Polymeric Scaffolds for the Regulation of Physical Cellular Microenvironments.Polymers (Basel). 2021 Nov 10;13(22):3880. doi: 10.3390/polym13223880. Polymers (Basel). 2021. PMID: 34833179 Free PMC article. Review.
References
-
- Park IH, Zhao R, West JA, Yabuuchi A, Huo HG, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451 141-U1. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Bianco P, Riminucci N, Kuznetsov S, Robey PG. Multipotential cells in the bone marrow stroma: Regulation in the context of organ physiology. Critical Reviews in Eukaryotic Gene Expression. 1999;9:159–173. - PubMed
-
- Prockop DJ. Marrow stromal cells as steam cells for nonhematopoietic tissues. Science. 1997;276:71–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

