The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity
- PMID: 21109192
- PMCID: PMC3683568
- DOI: 10.1016/j.cmet.2010.11.011
The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity
Abstract
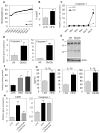
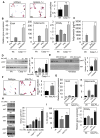
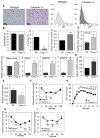
Obesity-induced inflammation originating from expanding adipose tissue interferes with insulin sensitivity. Important metabolic effects have been recently attributed to IL-1β and IL-18, two members of the IL-1 family of cytokines. Processing of IL-1β and IL-18 requires cleavage by caspase-1, a cysteine protease regulated by a protein complex called the inflammasome. We demonstrate that the inflammasome/caspase-1 governs adipocyte differentiation and insulin sensitivity. Caspase-1 is upregulated during adipocyte differentiation and directs adipocytes toward a more insulin-resistant phenotype. Treatment of differentiating adipocytes with recombinant IL-1β and IL-18, or blocking their effects by inhibitors, reveals that the effects of caspase-1 on adipocyte differentiation are largely conveyed by IL-1β. Caspase-1 and IL-1β activity in adipose tissue is increased both in diet-induced and genetically induced obese animal models. Conversely, mice deficient in caspase-1 are more insulin sensitive as compared to wild-type animals. In addition, differentiation of preadipocytes isolated from caspase-1(-/-) or NLRP3(-/-) mice resulted in more metabolically active fat cells. In vivo, treatment of obese mice with a caspase-1 inhibitor significantly increases their insulin sensitivity. Indirect calorimetry analysis revealed higher fat oxidation rates in caspase-1(-/-) animals. In conclusion, the inflammasome is an important regulator of adipocyte function and insulin sensitivity, and caspase-1 inhibition may represent a novel therapeutic target in clinical conditions associated with obesity and insulin resistance.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Dumasy V, Delhaye M, Cotton F, Deviere J. Fat malabsorption screening in chronic pancreatitis. Am J Gastroenterol. 2004;99:1350–1354. - PubMed
-
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. - PubMed
-
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. - PubMed
-
- He F, Doucet JA, Stephens JM. Caspase-mediated degradation of PPARgamma proteins in adipocytes. Obesity. 2008;16:1735–1741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

