Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab
- PMID: 21109694
- PMCID: PMC3031697
- DOI: 10.3324/haematol.2010.030759
Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab
Abstract
Background: In our efforts to develop novel effective treatment regimens for multiple myeloma we evaluated the potential benefits of combining the immunomodulatory drug lenalidomide with daratumumab. Daratumumab is a novel human CD38 monoclonal antibody which kills CD38+ multiple myeloma cells via antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity and apoptosis.
Design and methods: To explore the effect of lenalidomide combined with daratumumab, we first carried out standard antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity assays in which the CD38+ multiple myeloma cell line UM-9 and primary multiple myeloma cells isolated from patients were used as target cells. We also tested the effect of lenalidomide on daratumumab-dependent cell-mediated-cytotoxicity and complement-dependent cytotoxicity of multiple myeloma cells directly in the bone marrow mononuclear cells of multiple myeloma patients. Finally, we determined the daratumumab-dependent cell-mediated cytotoxicity using peripheral blood mononuclear cells of multiple myeloma patients receiving lenalidomide treatment.
Results: Daratumumab-dependent cell-mediated cytotoxicity of purified primary multiple myeloma cells, as well as of the UM-9 cell line, was significantly augmented by lenalidomide pre-treatment of the effector cells derived from peripheral blood mononuclear cells from healthy individuals. More importantly, we demonstrated a clear synergy between lenalidomide and daratumumab-induced antibody-dependent cell-mediated cytotoxicity directly in the bone marrow mononuclear cells of multiple myeloma patients, indicating that lenalidomide can also potentiate the daratumumab-dependent lysis of myeloma cells by activating the autologous effector cells within the natural environment of malignant cells. Finally, daratumumab-dependent cell-mediated cytotoxicity was significantly up-regulated in peripheral blood mononuclear cells derived from 3 multiple myeloma patients during lenalidomide treatment.
Conclusions: Our results indicate that powerful and complementary effects may be achieved by combining lenalidomide and daratumumab in the clinical management of multiple myeloma.
Figures
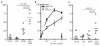



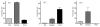
References
-
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. - PubMed
-
- Kale V, List AF. Immunomodulatory drugs (IMiDs): a new treatment option for myelodysplastic syndromes. Curr Pharm Biotechnol. 2006;7(5):339–42. - PubMed
-
- Knight R. IMiDs: a novel class of immunomodulators. Semin Oncol. 2005;32 (4 Suppl 5):S24–S30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

