Hsp90 inhibition increases p53 expression and destabilizes MYCN and MYC in neuroblastoma
- PMID: 21109931
- PMCID: PMC3212671
Hsp90 inhibition increases p53 expression and destabilizes MYCN and MYC in neuroblastoma
Abstract
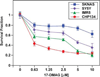
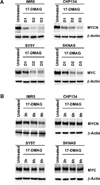
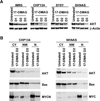
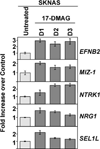
Neuroblastoma is a childhood cancer that exhibits either a favorable or an unfavorable phenotype. MYCN and MYC are oncoproteins that play crucial roles in determining the malignancy of unfavorable neuroblastoma. The Hsp90 superchaperone complex assists in the folding and function of a variety of oncogenic client proteins. Inhibition of Hsp90 by small molecule inhibitors leads to the destabilization of these oncogenic proteins and consequently suppresses tumor malignancy. Nonetheless, little is known about the effect of Hsp90 inhibition on the stability of MYCN and MYC proteins. In this study, we investigated the effect of Hsp90 inhibition on the phenotype of unfavorable neuroblastoma cells including its effect on MYCN and MYC expression. Two MYCN-amplified neuroblastoma cell lines (IMR5 and CHP134) and two non-MYCN-amplified cell lines (SY5Y and SKNAS) were used to address the effect of Hsp90 inhibition on the malignant phenotype of neuroblastoma. It was found that Hsp90 inhibition in neuroblastoma cell lines resulted in significant growth suppression, a decrease in MYCN and MYC expression, and an increase in the expression of p53. In the TP53-mutated SKNAS cell line, Hsp90 inhibition enhanced the expression of the favorable neuroblastoma genes EFNB2, MIZ-1 and NTRK1 (TrkA). In addition, Hsp90 inhibition reduced HDAC6 expression and enhanced tubulin acetylation. Together our data suggest that Hsp90 inhibition suppresses the growth of neuroblastoma through multiple cellular pathways and that MYC/MYCN destabilization is among the important consequences of Hsp90 inhibition.
Figures





References
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. - PubMed
-
- Modak S, Cheung NK. Neuroblastoma: Therapeutic strategies for a clinical enigma. Cancer Treat Rev. 2010;36:307–317. - PubMed
-
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. - PubMed
-
- Schwab M, Varmus HE, Bishop JM, et al. Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature. 1984;308:288–291. - PubMed
-
- Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. New Engl J Med. 1985;313:1111–1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

