IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function
- PMID: 21110318
- PMCID: PMC3373225
- DOI: 10.1002/eji.201041037
IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function
Abstract
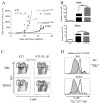
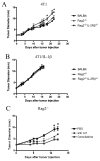
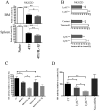
Chronic inflammation is associated with promotion of malignancy and tumor progression. Many tumors enhance the accumulation of myeloid-derived suppressor cells (MDSC), which contribute to tumor progression and growth by suppressing anti-tumor immune responses. Tumor-derived IL-1β secreted into the tumor microenvironment has been shown to induce the accumulation of MDSC possessing an enhanced capacity to suppress T cells. In this study, we found that the enhanced suppressive potential of IL-1β-induced MDSC was due to the activity of a novel subset of MDSC lacking Ly6C expression. This subset was present at low frequency in tumor-bearing mice in the absence of IL-1β-induced inflammation; however, under inflammatory conditions, Ly6C(neg) MDSC were predominant. Ly6C(neg) MDSC impaired NK cell development and functions in vitro and in vivo. These results identify a novel IL-1β-induced subset of MDSC with unique functional properties. Ly6C(neg) MDSC mediating NK cell suppression may thus represent useful targets for therapeutic interventions.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures


Comment in
-
The growing diversity and spectrum of action of myeloid-derived suppressor cells.Eur J Immunol. 2010 Dec;40(12):3317-20. doi: 10.1002/eji.201041170. Eur J Immunol. 2010. PMID: 21110315
References
-
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. - PubMed
-
- Apte RN, Voronov E. Is interleukin-1 a good or bad ‘guy’ in tumor immunobiology and immunotherapy? Imunol Rev. 2008;222:222–241. - PubMed
-
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. - PubMed

