THAP5 is a DNA-binding transcriptional repressor that is regulated in melanoma cells during DNA damage-induced cell death
- PMID: 21110952
- PMCID: PMC3034447
- DOI: 10.1016/j.bbrc.2010.11.092
THAP5 is a DNA-binding transcriptional repressor that is regulated in melanoma cells during DNA damage-induced cell death
Abstract
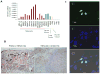
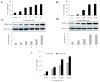
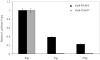
THAP5 was originally isolated as a specific interactor and substrate of the mitochondrial pro-apoptotic Omi/HtrA2 protease. It is a human zinc finger protein characterized by a restricted pattern of expression and the lack of orthologs in mouse and rat. The biological function of THAP5 is unknown but our previous studies suggest it could regulate G2/M transition in kidney cells and could be involved in human cardiomyocyte cell death associated with coronary artery disease (CAD). In this report, we expanded our studies on the properties and function of THAP5 in human melanoma cells. THAP5 was expressed in primary human melanocytes as well as in all melanoma cell lines that were tested. THAP5 protein level was significantly induced by UV irradiation or cisplatin treatment, conditions known to cause DNA damage. The induction of THAP5 correlated with a significant increase in apoptotic cell death. In addition, we show that THAP5 is a nuclear protein that could recognize and bind a specific DNA motif. THAP5 could also repress the transcription of a reporter gene in a heterologous system. Our work suggests that THAP5 is a DNA-binding protein and a transcriptional repressor. Furthermore, THAP5 has a pro-apoptotic function and it was induced in melanoma cells under conditions that promoted cell death.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Roussigne M, Kossida S, Lavigne AC, Clouaire T, Ecochard V, Glories A, Amalric F, Girard JP. The THAP domain: a novel protein motif with similarity to the DNA-binding domain of P element transposase. Trends Biochem Sci. 2003;28:66–69. - PubMed
-
- Cayrol C, Lacroix C, Mathe C, Ecochard V, Ceribelli M, Loreau E, Lazar V, Dessen P, Mantovani R, Aguilar L, Girard JP. The THAP-zinc finger protein THAP1 regulates endothelial cell proliferation through modulation of pRB/E2F cell-cycle target genes. Blood. 2007;109:584–594. - PubMed
-
- Deiss LP, Feinstein E, Berissi H, Cohen O, Kimchi A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 1995;9:15–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

