The temporal and spatial expression patterns of ABCG2 in the developing human heart
- PMID: 21111494
- PMCID: PMC4490625
- DOI: 10.1016/j.ijcard.2010.10.025
The temporal and spatial expression patterns of ABCG2 in the developing human heart
Abstract
Background: The discovery that the adult heart is not a terminally differentiated organ and contains stem/progenitor cells has important implications for the development of cellular therapeutics to treat heart disease. Moreover the discovery of cardiac stem cells might be important in furthering our understanding of both normal and abnormal cardiac development and yet little is known about these cell populations in the developing human heart, which we have focused on in this study.
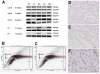
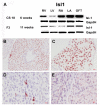
Methods: The presence of ABCG2 and islet-1 expressing cells in human heart was determined using immunohistochemistry and RT-PCR (and western blotting for ABCG2). Cardiac SP cells were isolated using FACS. Co-localisation immunohistochemistry was used to determine if ABCG2 positive cells expressed other known stem/progenitor cell, endothelial markers or cardiac markers.
Results: We observed that ABCG2 expressing cells show a difference in both their temporal and spatial patterns of expression from Islet-1 expressing cardiac progenitors. We identified rare cells that expressed both ABCG2 and markers of other cell lineages including CD31, CD34 and alpha-actinin. We also noted the presence of cells that only expressed ABCG2. We isolated cardiac SP cells and confirmed the SP cell phenotype.
Conclusions: Our results suggest that the developing human heart contains at least two distinct cardiac stem/progenitor cell populations one of which, the ABCG2 positive cells, can be readily isolated, suggesting that this tissue could be a useful source of cardiac stem cells.
Copyright © 2010 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Beltrami A, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
-
- Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. - PubMed
-
- Martin CM, Meeson AP, Roberston SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, Identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

