Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy
- PMID: 21111744
- PMCID: PMC3035763
- DOI: 10.1016/j.yjmcc.2010.11.012
Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy
Abstract
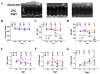
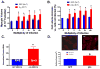
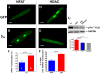
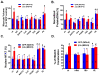
Pathological cardiac hypertrophy (PCH) is associated with the development of arrhythmia and congestive heart failure. While calcium (Ca(2+)) is implicated in hypertrophic signaling pathways, the specific role of Ca(2+) influx through the L-type Ca(2+) channel (I(Ca-L)) has been controversial and is the topic of this study. To determine if and how sustained increases in I(Ca-L) induce PCH, transgenic mouse models with low (LE) and high (HE) expression levels of the β2a subunit of Ca(2+) channels (β2a) and in cultured adult feline (AF) and neonatal rat (NR) ventricular myocytes (VMs) infected with an adenovirus containing a β2a-GFP were used. In vivo, β2a LE and HE mice had increased heart weight to body weight ratio, posterior wall and interventricular septal thickness, tissue fibrosis, myocyte volume, and cross-sectional area and the expression of PCH markers in a time- and dose-dependent manner. PCH was associated with a hypercontractile phenotype including enhanced I(Ca-L), fractional shortening, peak Ca(2+) transient, at the myocyte level, greater ejection fraction, and fractional shortening at the organ level. In addition, LE mice had an exaggerated hypertrophic response to transverse aortic constriction. In vitro overexpression of β2a in cultured AFVMs increased I(Ca-L), cell volume, protein synthesis, NFAT, and HDAC translocations and in NRVMs increased surface area. These effects were abolished by the blockade of I(Ca-L), intracellular Ca(2+), calcineurin, CaMKII, and SERCA. In conclusion, increasing I(Ca-L) is sufficient to induce PCH through the calcineurin/NFAT and CaMKII/HDAC pathways. Both cytosolic and SR/ER-nuclear envelop Ca(2+) pools were shown to be involved.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures







References
-
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006 Aug;7(8):589–600. - PubMed
-
- Bristow MR, Kantrowitz NE, Ginsburg R, Fowler MB. Beta-adrenergic function in heart muscle disease and heart failure. J Mol Cell Cardiol. 1985 Jul;17( Suppl 2):41–52. - PubMed
-
- Dorn GW., 2nd Adrenergic pathways and left ventricular remodeling. J Card Fail. 2002 Dec;8(6 Suppl):S370–3. - PubMed
-
- Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005 Apr;11(4):409–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

