The O-glycosylated linker from the Trichoderma reesei Family 7 cellulase is a flexible, disordered protein
- PMID: 21112302
- PMCID: PMC2998629
- DOI: 10.1016/j.bpj.2010.10.032
The O-glycosylated linker from the Trichoderma reesei Family 7 cellulase is a flexible, disordered protein
Abstract
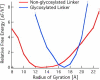
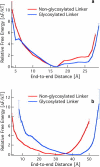
Fungi and bacteria secrete glycoprotein cocktails to deconstruct cellulose. Cellulose-degrading enzymes (cellulases) are often modular, with catalytic domains for cellulose hydrolysis and carbohydrate-binding modules connected by linkers rich in serine and threonine with O-glycosylation. Few studies have probed the role that the linker and O-glycans play in catalysis. Since different expression and growth conditions produce different glycosylation patterns that affect enzyme activity, the structure-function relationships that glycosylation imparts to linkers are relevant for understanding cellulase mechanisms. Here, the linker of the Trichoderma reesei Family 7 cellobiohydrolase (Cel7A) is examined by simulation. Our results suggest that the Cel7A linker is an intrinsically disordered protein with and without glycosylation. Contrary to the predominant view, the O-glycosylation does not change the stiffness of the linker, as measured by the relative fluctuations in the end-to-end distance; rather, it provides a 16 Å extension, thus expanding the operating range of Cel7A. We explain observations from previous biochemical experiments in the light of results obtained here, and compare the Cel7A linker with linkers from other cellulases with sequence-based tools to predict disorder. This preliminary screen indicates that linkers from Family 7 enzymes from other genera and other cellulases within T. reesei may not be as disordered, warranting further study.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Spiro R.G. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. - PubMed
-
- Goto M. Protein O-glycosylation in fungi: diverse structures and multiple functions. Biosci. Biotechnol. Biochem. 2007;71:1415–1427. - PubMed
-
- Wormald M.R., Petrescu A.J., Dwek R.A. Conformational studies of oligosaccharides and glycopeptides: complementarity of NMR, X-ray crystallography, and molecular modelling. Chem. Rev. 2002;102:371–386. - PubMed
-
- Deshpande N., Wilkins M.R., Nevalainen H. Protein glycosylation pathways in filamentous fungi. Glycobiology. 2008;18:626–637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

