Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown
- PMID: 21112944
- PMCID: PMC3043817
- DOI: 10.1152/ajplung.00304.2010
Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown
Abstract
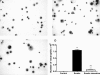
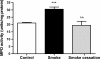
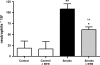
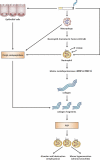
There is increasing evidence that the neutrophil chemoattractant proline-glycine-proline (PGP), derived from the breakdown of the extracellular matrix, plays an important role in neutrophil recruitment to the lung. PGP formation is a multistep process involving neutrophils, metalloproteinases (MMPs), and prolyl endopeptidase (PE). This cascade of events is now investigated in the development of lung emphysema. A/J mice were whole body exposed to cigarette smoke for 20 wk. After 20 wk or 8 wk after smoking cessation, animals were killed, and bronchoalveolar lavage fluid and lung tissue were collected to analyze the neutrophilic airway inflammation, the MMP-8 and MMP-9 levels, the PE activity, and the PGP levels. Lung tissue degradation was assessed by measuring the mean linear intercept. Additionally, we investigated the effect of the peptide L-arginine-threonine-arginine (RTR), which binds to PGP sequences, on the smoke-induced neutrophil influx in the lung after 5 days of smoke exposure. Neutrophilic airway inflammation was induced by cigarette smoke exposure. MMP-8 and MMP-9 levels, PE activity, and PGP levels were elevated in the lungs of cigarette smoke-exposed mice. PE was highly expressed in epithelial and inflammatory cells (macrophages and neutrophils) in lung tissue of cigarette smoke-exposed mice. After smoking cessation, the neutrophil influx, the MMP-8 and MMP-9 levels, the PE activity, and the PGP levels were decreased or reduced to normal levels. Moreover, RTR inhibited the smoke-induced neutrophil influx in the lung after 5 days' smoke exposure. In the present murine model of cigarette smoke-induced lung emphysema, it is demonstrated for the first time that all relevant components (neutrophils, MMP-8, MMP-9, PE) involved in PGP formation from collagen are upregulated in the airways. Together with MMPs, PE may play an important role in the formation of PGP and thus in the pathophysiology of lung emphysema.
Figures




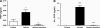
References
-
- Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis 12: 361–367, 2008 - PubMed
-
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 28: 12–24, 2003 - PubMed
-
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 343: 269–280, 2000 - PubMed
-
- Barrett AJ, Rawlings ND. Oligopeptidases, and the emergence of the prolyl oligopeptidase family. Biol Chem Hoppe Seyler 373: 353–360, 1992 - PubMed
-
- Belvisi MG, Bottomley KM. The role of matrix metalloproteinases (MMPs) in the pathophysiology of chronic obstructive pulmonary disease (COPD): a therapeutic role for inhibitors of MMPs? Inflamm Res 52: 95–100, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

