Metalloprotease type III effectors that specifically cleave JNK and NF-κB
- PMID: 21113130
- PMCID: PMC3020117
- DOI: 10.1038/emboj.2010.297
Metalloprotease type III effectors that specifically cleave JNK and NF-κB
Abstract
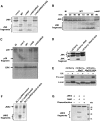
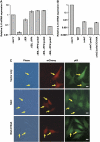
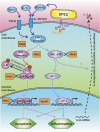
Two major arms of the inflammatory response are the NF-κB and c-Jun N-terminal kinase (JNK) pathways. Here, we show that enteropathogenic Escherichia coli (EPEC) employs the type III secretion system to target these two signalling arms by injecting host cells with two effector proteins, NleC and NleD. We provide evidence that NleC and NleD are Zn-dependent endopeptidases that specifically clip and inactivate RelA (p65) and JNK, respectively, thus blocking NF-κB and AP-1 activation. We show that NleC and NleD co-operate and complement other EPEC effectors in accomplishing maximal inhibition of IL-8 secretion. This is a remarkable example of a pathogen using multiple effectors to manipulate systematically the host inflammatory response signalling network.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



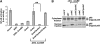

References
-
- Barkett M, Gilmore TD (1999) Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 18: 6910–6924 - PubMed
-
- Chen FE, Huang DB, Chen YQ, Ghosh G (1998a) Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature 391: 410–413 - PubMed
-
- Chen YQ, Ghosh S, Ghosh G (1998b) A novel DNA recognition mode by the NF-kappa B p65 homodimer. Nat Struct Biol 5: 67–73 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

