(+)-Discodermolide: Total Synthesis, Construction of Novel Analogues, and Biological Evaluation
- PMID: 21113402
- PMCID: PMC2991168
- DOI: 10.1016/j.tet.2007.10.039
(+)-Discodermolide: Total Synthesis, Construction of Novel Analogues, and Biological Evaluation
Figures
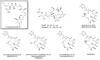


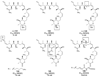


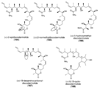




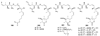


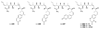


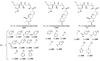


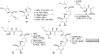







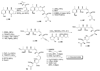
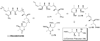
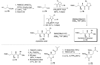










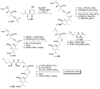
References
-
- Mann J. Nat. Rev. Cancer. 2002;2:143–148. - PubMed
-
- Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665–667. - PubMed
- Schiff PB, Horwitz SB. Proc. Natl. Acad. Sci. U. S. A. 1980;77:1561–1565. - PMC - PubMed
- Parness J, Horwitz SB. J. Cell Biol. 1981;91:479–487. - PMC - PubMed
- Horwitz SB, Parness J, Schiff PB, Manfredi JJ. Cold Spring Harbor Symp. Quant. Biol. 1982;46:219–226. - PubMed
-
- Gerth K, Bedorf N, Hofle G, Irschik H, Reichenbach H. J. Antibiot. 1996;49:560–563. - PubMed
-
- Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Cancer Res. 1995;55:2325–2333. - PubMed
- Su D-S, Balog A, Meng D, Bertinato P, Danishefsky SJ, Zheng Y-H, Chou T-C, He L, Horwitz SB. Angew. Chem., Int. Engl. 1997;36:2093–2096.
- Chou T-C, Zhang X-G, Balog A, Su D-S, Meng D, Savin K, Bertino JR, Danishefsky SJ. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9642–9647. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
