Fixing ryanodine receptor Ca leak - a novel therapeutic strategy for contractile failure in heart and skeletal muscle
- PMID: 21113427
- PMCID: PMC2989530
- DOI: 10.1016/j.ddmec.2010.09.009
Fixing ryanodine receptor Ca leak - a novel therapeutic strategy for contractile failure in heart and skeletal muscle
Abstract
A critical component in regulating cardiac and skeletal muscle contractility is the release of Ca(2+) via ryanodine receptor (RyR) Ca(2+) release channels in the sarcoplasmic reticulum (SR). In heart failure and myopathy, the RyR has been found to be excessively phosphorylated or nitrosylated and depleted of the RyR-stabilizing protein calstabin (FK506 binding protein 12/12.6). This remodeling of the RyR channel complex results in an intracellular SR Ca(2+) leak and impaired contractility. Despite recent advances in heart failure treatment, there are still devastatingly high mortality rates with this disease. Moreover, pharmacological treatment for muscle weakness and myopathy is nearly nonexistent. A novel class of RyR-stabilizing drugs, rycals, which reduce Ca(2+) leak by stabilizing the RyR channels due to preservation of the RyR-calstabin interaction, have recently been shown to improve contractile function in both heart and skeletal muscle. This opens up a novel therapeutic strategy for the treatment of contractile failure in the cardiac and skeletal muscle.
Conflict of interest statement
Conflict of interest: A.R. Marks is a consultant for a start-up company, ARMGO Pharma Inc., that is targeting RyR channels to treat heart disease and to improve exercise capacity in muscle diseases
Figures

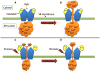
References
-
- Farr MA, Basson CT. Sparking the failing heart. N Engl J Med. 2004;351 (2):185–187. - PubMed
-
- Lloyd-Jones D, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121(7):e46–e215. - PubMed
-
- Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101 (4):365–376. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
