Free radical formation in novel carotenoid metal ion complexes of astaxanthin
- PMID: 21114306
- PMCID: PMC3018833
- DOI: 10.1021/jp109039v
Free radical formation in novel carotenoid metal ion complexes of astaxanthin
Abstract
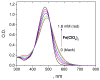
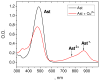
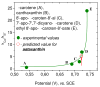
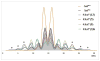
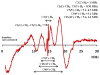
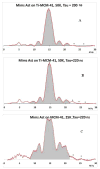
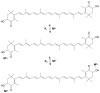
The carotenoid astaxanthin forms novel metal ion complexes with Ca(2+), Zn(2+), and Fe(2+). MS and NMR measurements indicate that the two oxygen atoms on the terminal cyclohexene ring of astaxanthin chelate the metal to form 1:1 complexes with Ca(2+) and Zn(2+) at low salt concentrations <0.2 mM. The stability constants of these complexes increased by a factor of 85 upon changing the solvent from acetonitrile to ethanol for Ca(2+) and by a factor of 7 for Zn(2+) as a consequence of acetonitrile being a part of the complex. Optical studies showed that at high concentrations (>0.2 mM) of salt, 2:1 metal/astaxanthin complexes were formed in ethanol. In the presence of Ca(2+) and Zn(2+), salts the lifetime of the radical cation and dication formed electrochemically decreased relative to those formed from the uncomplexed carotenoid. DFT calculations showed that the deprotonation of the radical cation at the carbon C3 position resulted in the lowest energy neutral radical, while proton loss at the C5, C9, or C13 methyl groups was less favorable. Pulsed EPR measurements were carried out on UV-produced radicals of astaxanthin supported on silica-alumina, MCM-41, or Ti-MCM-41. The pulsed EPR measurements detected the radical cation and neutral radicals formed by proton loss at 77 K from the C3, C5, C9, and C13 methyl groups and a radical anion formed by deprotonation of the neutral radical at C3. There was more than an order of magnitude increase in the concentration of radicals on Ti-MCM-41 relative to MCM-41, and the radical cation concentration exceeded that of the neutral radicals.
Figures






Similar articles
-
EPR study of the astaxanthin n-octanoic acid monoester and diester radicals on silica-alumina.J Phys Chem B. 2012 Nov 8;116(44):13200-10. doi: 10.1021/jp307421e. Epub 2012 Oct 25. J Phys Chem B. 2012. PMID: 23039790
-
Structure and properties of 9'-cis neoxanthin carotenoid radicals by electron paramagnetic resonance measurements and density functional theory calculations: present in LHC II?J Phys Chem B. 2009 Apr 30;113(17):6087-96. doi: 10.1021/jp810604s. J Phys Chem B. 2009. PMID: 19344105
-
Electrochemical study of astaxanthin and astaxanthin n-octanoic monoester and diester: tendency to form radicals.J Phys Chem B. 2014 Mar 6;118(9):2331-9. doi: 10.1021/jp4121436. Epub 2014 Feb 21. J Phys Chem B. 2014. PMID: 24494596
-
Chemistry of carotenoid neutral radicals.Arch Biochem Biophys. 2015 Apr 15;572:167-174. doi: 10.1016/j.abb.2015.02.005. Epub 2015 Feb 14. Arch Biochem Biophys. 2015. PMID: 25687648 Review.
-
Photo Protection of Haematococcus pluvialis Algae by Astaxanthin: Unique Properties of Astaxanthin Deduced by EPR, Optical and Electrochemical Studies.Antioxidants (Basel). 2017 Oct 21;6(4):80. doi: 10.3390/antiox6040080. Antioxidants (Basel). 2017. PMID: 29065482 Free PMC article. Review.
Cited by
-
The metal cation chelating capacity of astaxanthin. Does this have any influence on antiradical activity?Molecules. 2012 Jan 20;17(1):1039-54. doi: 10.3390/molecules17011039. Molecules. 2012. PMID: 22267192 Free PMC article.
-
Quantification and Improvement of the Dynamics of Human Serum Albumin and Glycated Human Serum Albumin with Astaxanthin/Astaxanthin-Metal Ion Complexes: Physico-Chemical and Computational Approaches.Int J Mol Sci. 2022 Apr 26;23(9):4771. doi: 10.3390/ijms23094771. Int J Mol Sci. 2022. PMID: 35563162 Free PMC article.
-
Skin Protection by Carotenoid Pigments.Int J Mol Sci. 2024 Jan 24;25(3):1431. doi: 10.3390/ijms25031431. Int J Mol Sci. 2024. PMID: 38338710 Free PMC article. Review.
-
Structures of Astaxanthin and Their Consequences for Therapeutic Application.Int J Food Sci. 2020 Jul 20;2020:2156582. doi: 10.1155/2020/2156582. eCollection 2020. Int J Food Sci. 2020. PMID: 32775406 Free PMC article. Review.
-
DFT and molecular dynamics studies of astaxanthin-metal ions (Cu2+ and Zn2+) complex to prevent glycated human serum albumin from possible unfolding.Heliyon. 2021 Mar 24;7(3):e06548. doi: 10.1016/j.heliyon.2021.e06548. eCollection 2021 Mar. Heliyon. 2021. PMID: 33851048 Free PMC article.
References
-
- Holt NE, Fleming GR, Niyogi KK. Biochemistry. 2004;43:8281–8289. - PubMed
-
- Pascal AA, Liu ZF, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang WR, Ruban A. Nature. 2005;436:134–137. - PubMed
-
- Mozzo M, Dall’Osto L, Hienerwadel R, Bassi R, Croce R. J Biol Chem. 2008;283:6184–6192. - PubMed
-
- Holt NE, Zigmantas D, Valkunas L, Li XP, Niyogi KK, Fleming GR. Science. 2005;307:433–436. - PubMed
-
- Ahn TK, Avenson TJ, Ballottari M, Cheng YC, Niyogi KK, Bassi R, Fleming GR. Science. 2008;320:794–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

