Axonal loss and neurofilament phosphorylation changes accompany lesion development and clinical progression in multiple sclerosis
- PMID: 21114565
- PMCID: PMC8094317
- DOI: 10.1111/j.1750-3639.2010.00466.x
Axonal loss and neurofilament phosphorylation changes accompany lesion development and clinical progression in multiple sclerosis
Abstract
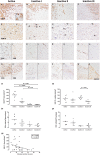
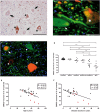
Neuroaxonal damage and loss are increasingly recognized as disability determining features in multiple sclerosis (MS) pathology. However, little is known about the long-term sequelae of inflammatory demyelination on neurons and axons. Spinal cord tissue of 31 MS patients was compared to three amyotrophic lateral sclerosis (ALS) and 10 control subjects. MS lesions were staged according to the density of KiM-1P positive macrophages and microglia and the presence of myelin basic protein (MBP) positive phagocytes. T cells were quantified in the parenchyma and meninges. Neuroaxonal changes were studied by immunoreactivity (IR) for amyloid precursor protein (APP) and variably phosphorylated neurofilaments (SMI312, SMI31, SMI32). Little T cell infiltration was still evident in chronic inactive lesions. The loss of SMI32 IR in ventral horn neurons correlated with MS lesion development and disease progression. Similarly, axonal loss in white matter (WM) lesions correlated with disease duration. A selective reduction of axonal phosphorylated neurofilaments (SMI31) was observed in WM lesions. In ALS, the loss of neuronal SMI32 IR was even more pronounced, whereas the relative axonal reduction resembled that found in MS. Progressive neuroaxonal neurofilament alterations in the context of chronic inflammatory demyelination may reflect changes in neuroaxonal metabolism and result in chronic neuroaxonal dysfunction as a putative substrate of clinical progression.
© 2011 The Authors; Brain Pathology © 2011 International Society of Neuropathology.
Figures

References
-
- Agosta F, Absinta M, Sormani MP, Ghezzi A, Bertolotto A, Montanari E et al (2007) In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain 130:2211–2219. - PubMed
-
- Bannerman PG, Hahn A, Ramirez S, Morley M, Bonnemann C, Yu S et al (2005) Motor neuron pathology in experimental autoimmune encephalomyelitis: studies in THY1‐YFP transgenic mice. Brain 128:1877–1886. - PubMed
-
- Barnett MH, Henderson AP, Prineas JW (2006) The macrophage in MS: just a scavenger after all? Pathology and pathogenesis of the acute MS lesion. Mult Scler 12:121–132. - PubMed
-
- Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W (2000) Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123(Pt 6):1174–1183. - PubMed
-
- Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD (2000) Neurological disability correlates with spinal cord axonal loss and reduced N‐acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol 48:893–901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

