Chromosome integrity at a double-strand break requires exonuclease 1 and MRX
- PMID: 21115410
- PMCID: PMC3031249
- DOI: 10.1016/j.dnarep.2010.10.004
Chromosome integrity at a double-strand break requires exonuclease 1 and MRX
Abstract
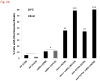
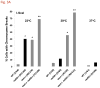
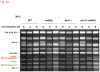
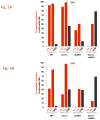
The continuity of duplex DNA is generally considered a prerequisite for chromosome continuity. However, as previously shown in yeast as well as human cells, the introduction of a double-strand break (DSB) does not generate a chromosome break (CRB) in yeast or human cells. The transition from DSB to CRB was found to be under limited control by the tethering function of the RAD50/MRE11/XRS2 (MRX) complex. Using a system for differential fluorescent marking of both sides of an endonuclease-induced DSB in single cells, we found that nearly all DSBs are converted to CRBs in cells lacking both exonuclease 1 (EXO1) activity and MRX complex. Thus, it appears that some feature of exonuclease processing or resection at a DSB is critical for maintaining broken chromosome ends in close proximity. In addition, we discovered a thermal sensitive (cold) component to CRB formation in an MRX mutant that has implications for chromosome end mobility and/or end-processing.
Published by Elsevier B.V.
Figures








References
-
- Mills KD, Ferguson DO, Alt FW. The role of DNA breaks in genomic instability and tumorigenesis. Immunol Rev. 2003;194:77–95. - PubMed
-
- Albertson DG, Collins C, McCormick F, Gray JW. Chromosome aberrations in solid tumors. Nat Genet. 2003;34:369–376. - PubMed
-
- Lobachev K, Vitriol E, Stemple J, Resnick MA, Bloom K. Chromosome fragmentation after induction of a double-strand break is an active process prevented by the RMX repair complex. Curr Biol. 2004;14:2107–2112. - PubMed
-
- Kaye JA, Melo JA, Cheung SK, Vaze MB, Haber JE, Toczyski DP. DNA breaks promote genomic instability by impeding proper chromosome segregation. Curr Biol. 2004;14:2096–2106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

