MCP-1 contributes to arteriovenous fistula failure
- PMID: 21115617
- PMCID: PMC3014033
- DOI: 10.1681/ASN.2010040373
MCP-1 contributes to arteriovenous fistula failure
Abstract
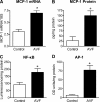
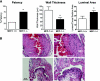
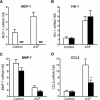
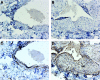
Vascular access dysfunction compromises the care of patients on chronic hemodialysis. Elucidating the mechanisms of such dysfunction and devising strategies that may interrupt neointimal hyperplasia and relevant pathogenetic pathways are essential. Here, we show that, in the venous segment of a murine model of an arteriovenous fistula, monocyte chemoattractant protein-1 (MCP-1) mRNA and protein increase, accompanied by increased activity of the transcription factors NF-κB and AP-1. Genetic deficiency of MCP-1 proved markedly protective in this murine model, reflected by increased fistula patency 6 weeks after its formation, decreased venous wall thickness, and increased luminal area. An early effect of MCP-1 deficiency was the attenuation of the marked induction of CCL5 (RANTES) that occurred in this model, a chemokine recently recognized as a critical participant in vascular injury. Finally, in a rat model of an arteriovenous fistula, we localized expression of MCP-1 to the endothelium, proliferating smooth muscle cells and infiltrating leukocytes. In summary, marked upregulation of MCP-1 occurs in the venous segment of an arteriovenous fistula in rodents, and this vasculopathic chemokine contributes to failure of the fistula.
Figures
References
-
- Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 - PubMed
-
- Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P: Biology of arteriovenous fistula failure. J Nephrol 20: 150–163, 2007 - PubMed
-
- Schwab SJ, Harrington JT, Singh A, Roher R, Shohaib SA, Perrone RD, Meyer K, Beasley D: Vascular access for hemodialysis. Kidney Int 55: 2078–2090, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

