Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide
- PMID: 21115704
- PMCID: PMC3028696
- DOI: 10.1128/AEM.01968-10
Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide
Abstract
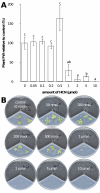
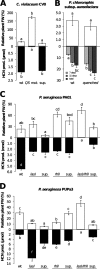
The volatile-mediated impact of bacteria on plant growth is well documented, and contrasting effects have been reported ranging from 6-fold plant promotion to plant killing. However, very little is known about the identity of the compounds responsible for these effects or the mechanisms involved in plant growth alteration. We hypothesized that hydrogen cyanide (HCN) is a major factor accounting for the observed volatile-mediated toxicity of some strains. Using a collection of environmental and clinical strains differing in cyanogenesis, as well as a defined HCN-negative mutant, we demonstrate that bacterial HCN accounts to a significant extent for the deleterious effects observed when growing Arabidopsis thaliana in the presence of certain bacterial volatiles. The environmental strain Pseudomonas aeruginosa PUPa3 was less cyanogenic and less plant growth inhibiting than the clinical strain P. aeruginosa PAO1. Quorum-sensing deficient mutants of C. violaceum CV0, P. aeruginosa PAO1, and P. aeruginosa PUPa3 showed not only diminished HCN production but also strongly reduced volatile-mediated phytotoxicity. The double treatment of providing plants with reactive oxygen species scavenging compounds and overexpressing the alternative oxidase AOX1a led to a significant reduction of volatile-mediated toxicity. This indicates that oxidative stress is a key process in the physiological changes leading to plant death upon exposure to toxic bacterial volatiles.
Figures


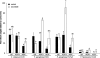
References
-
- Alstrom, S., and R. G. Burns. 1989. Cyanide production by rhizobacteria as a possible mechanism of plant-growth inhibition. Biol. Fert. Soils 7:232-238.
-
- Antunes, L. C. M., R. B. R. Ferreira, M. M. C. Buckner, and B. B. Finlay. 2010. Quorum sensing in bacterial virulence. Microbiol. SGM 156:2271-2282. - PubMed
-
- Astrom, B. 1991. Role of bacterial cyanide production in differential reaction of plant cultivars to deleterious rhizosphere pseudomonads. Plant Soil 133:93-100.
-
- Bakker, A. W., and B. Schippers. 1987. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas sp.-mediated plant growth stimulation. Soil Biol. Biochem. 19:451-457.
-
- Blumer, C., and D. Haas. 2000. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol. 173:170-177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

