Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells
- PMID: 21115743
- PMCID: PMC3059727
- DOI: 10.1158/1541-7786.MCR-10-0398
Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells
Abstract
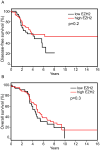
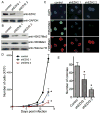
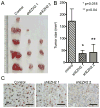
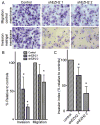
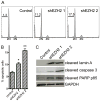
Enhancer of zeste homolog 2 (EZH2) is the catalytic subunit of the polycomb repressive complex 2 (PRC2) that includes noncatalytic subunits suppressor of zeste 12 (SUZ12) and embryonic ectoderm development (EED). When present in PRC2, EZH2 catalyzes trimethylation on lysine 27 residue of histone H3 (H3K27Me3), resulting in epigenetic silencing of gene expression. Here, we investigated the expression and function of EZH2 in epithelial ovarian cancer (EOC). When compared with primary human ovarian surface epithelial (pHOSE) cells, EZH2, SUZ12, and EED were expressed at higher levels in all 8 human EOC cell lines tested. Consistently, H3K27Me3 was also overexpressed in human EOC cell lines compared with pHOSE cells. EZH2 was significantly overexpressed in primary human EOCs (n = 134) when compared with normal ovarian surface epithelium (n = 46; P < 0.001). EZH2 expression positively correlated with expression of Ki67 (P < 0.001; a marker of cell proliferation) and tumor grade (P = 0.034) but not tumor stage (P = 0.908) in EOC. There was no correlation of EZH2 expression with overall (P = 0.3) or disease-free survival (P = 0.2) in high-grade serous histotype EOC patients (n = 98). Knockdown of EZH2 expression reduced the level of H3K27Me3 and suppressed the growth of human EOC cells both in vitro and in vivo in xenograft models. EZH2 knockdown induced apoptosis of human EOC cells. Finally, we showed that EZH2 knockdown suppressed the invasion of human EOC cells. Together, these data demonstrate that EZH2 is frequently overexpressed in human EOC cells and its overexpression promotes the proliferation and invasion of human EOC cells, suggesting that EZH2 is a potential target for developing EOC therapeutics.
©2010 AACR.
Conflict of interest statement
No potential conflicts of interest were declared.
Figures

References
-
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. - PubMed
-
- Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–96. - PubMed
-
- Muller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197–208. - PubMed
-
- Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell. 2004;15:57–67. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

