In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor
- PMID: 21115794
- PMCID: PMC3028777
- DOI: 10.1128/AAC.01209-10
In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor
Abstract
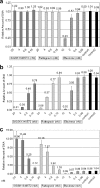
S/GSK1349572 is a next-generation HIV integrase (IN) inhibitor designed to deliver potent antiviral activity with a low-milligram once-daily dose requiring no pharmacokinetic (PK) booster. In addition, S/GSK1349572 demonstrates activity against clinically relevant IN mutant viruses and has potential for a high genetic barrier to resistance. S/GSK1349572 is a two-metal-binding HIV integrase strand transfer inhibitor whose mechanism of action was established through in vitro integrase enzyme assays, resistance passage experiments, activity against viral strains resistant to other classes of anti-HIV agents, and mechanistic cellular assays. In a variety of cellular antiviral assays, S/GSK1349572 inhibited HIV replication with low-nanomolar or subnanomolar potency and with a selectivity index of 9,400. The protein-adjusted half-maximal effective concentration (PA-EC(50)) extrapolated to 100% human serum was 38 nM. When virus was passaged in the presence of S/GSK1349572, highly resistant mutants were not selected, but mutations that effected a low fold change (FC) in the EC(50) (up to 4.1 fold) were identified in the vicinity of the integrase active site. S/GSK1349572 demonstrated activity against site-directed molecular clones containing the raltegravir-resistant signature mutations Y143R, Q148K, N155H, and G140S/Q148H (FCs, 1.4, 1.1, 1.2, and 2.6, respectively), while these mutants led to a high FC in the EC(50) of raltegravir (11- to >130-fold). Either additive or synergistic effects were observed when S/GSK1349572 was tested in combination with representative approved antiretroviral agents; no antagonistic effects were seen. These findings demonstrate that S/GSK1349572 would be classified as a next-generation drug in the integrase inhibitor class, with a resistance profile markedly different from that of first-generation integrase inhibitors.
Figures


References
-
- Billich, A. 2003. S-1360 Shionogi-GlaxoSmithKline. Curr. Opin. Investig. Drugs 4:206-209. - PubMed
-
- Boros, E. E., B. A. Johns, E. P. Garvey, C. S. Koble, and W. H. Miller. 2006. Synthesis and HIV-integrase strand transfer inhibition activity of 7-hydroxy[1,3]thiazolo[5,4-b]pyridin-5(4H)-ones. Bioorg. Med. Chem. Lett. 16:5668-5672. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases

